Scroll to:
Thermodynamic modeling of interphase distribution of chromium and boron in slags of AOD reduction period
https://doi.org/10.17073/0368-0797-2024-3-351-359
Abstract
The paper presents the results of a thermodynamic modeling of the chromium and boron reduction from slags of reduction period of argon-oxygen decarburization (AOD) by a complex reducing agent containing silicon and aluminum. Using the simplex lattice method, an experiment planning matrix is constructed containing 16 compositions of the oxide system СаО – SiO2 – (3 – 6 %) В2О3 – 12 % Cr2O3 – 3 % Al2O3 – 8 % MgO of variable basicity 1.0 – 2.5. The results of thermodynamic modeling are graphically presented in form of dependence of equilibrium distribution of chromium and boron on the slag composition at temperatures of 1600 and 1700 °C. The constructed diagrams make it possible to quantify the influence of the temperature, basicity and B2O3 in the slag on equilibrium interphase distribution of chromium and boron. It is established that increasing the slag basicity from 1.0 to 2.5 improves the process of chromium reduction, but restores the boron stability. With an increase in B2O3 content in the slag, a slight deterioration of chromium reduction process occurs, while the boron content in the metal increases. With a simultaneous increase in basicity up to 2.5 and a decrease in boron oxide in the slag from 5 to 3 %, the interphase distribution coefficient of chromium is reduced to 1.5·10–3. Changing the process temperature from 1600 to 1700 °C does not have a negative effect on the process of chromium reduction, but worsens the boron reduction conditions. Based on analysis of the formed slag phases and thermodynamics of the reactions of their formation, it is established that chromium is mainly reduced by aliminum with only partial development of silicothermy. The residual silicon content reduces boron, thereby limiting its concentration in the metal. The results of high-temperature experiments showed high correspondence with the results of thermodynamic studies.
Keywords
For citations:
Babenko A.A., Zhuchkov V.I., Kel’ N.I., Upolovnikova A.G., Shartdinov R.R. Thermodynamic modeling of interphase distribution of chromium and boron in slags of AOD reduction period. Izvestiya. Ferrous Metallurgy. 2024;67(3):351-359. https://doi.org/10.17073/0368-0797-2024-3-351-359
Introduction
Stainless steel is an absolutely essential part of a modern economy – the annually growing volumes of its consumption and a wide range of applications from medical products [1] to structural materials prove this statement1. Stainless steel is so popular because it is resistant to corrosion in various aggressive environments as an oxide layer, with a high concentration of chromium (12 wt. % and higher), forms on the metal surface, which prevents the steel from contacting air oxygen [2 – 4]. Despite the obvious advantages of stainless steel, the domestic production volumes are modest and the demand for this steel is covered by imports [5].
Currently, the main method of producing low-carbon stainless steel is the duplex process with smelting carbonaceous semi-product (1.5 – 2.0 wt. % C) in an arc furnace followed by treatment in the argon-oxygen decarburization (AOD) unit [6; 7]. The AOD process includes two periods: oxidation and reduction. During the oxidation period, the carbonaceous semi-product of stainless steel is decarbonized by blowing a mixture of oxygen and argon through it. When the carbon concentration in the metal drops to 0.03 wt. % or less, the reduction period of melting begins, during which the bath of the unit is purged with nothing but argon, and lime, ferroalloys (ferrosilicon, ferrosilicochrome) and сalcium fluoride are added [8].
As a result of chromium oxidation by oxygen, the concentration of Cr2O3 in the slag increases, which negatively affects the technological processes occurring in the reduction period of melting, the intensity of their development being limited by the viscosity of the formed oxide system. According to [9], Cr2O3 usually has low solubility (5 %) in CaO – SiO2 – Al2O3 – MgO based slags, which increases their melting point and, consequently, viscosity. Therefore, calcium fluoride (CaF2 ) is used to reduce the viscosity of slags in the reduction period of melting. The use of this flux has the following downsides: it is environmentally unfriendly as volatile carcinogenic fluorine compounds are formed, physical properties of the formed slags are inconsistent and the effect of silicate decomposition of solid slags persists during storage. Therefore, it is reasonable to explore other liquefying additives to replace calcium fluoride, for example, pegmatite [10], Al2O3 [11] or B2O3 [12]. Although the use of Al2O3 prevents the formation of volatile fluoride compounds, according to [11], the refining ability of the slag deteriorates. As such, its use is limited. Therefore, the boron-containing material is a reasonable choice as it is an inexpensive, available and environmentally friendly fluxing material.
Although B2O3 is an acidic oxide and facilitates polymerization [13], it helps to reduce slag viscosity by changing the structural components of the melt mesh. Adding B2O3 to the slag helps to improve kinetics of chromium reduction and metal desulfurization [14; 15]. Hardening characteristics of low-carbon steel are to improve and the aging effect is to reduce [17] due to expected partial reduction of boron by silicon and aluminum dissolved in the steel, followed by its transfer to the metal, in addition to the slag liquefying with boron oxide [17].
Practically no domestic or foreign researchers have explored the effectiveness of the interphase distribution of chromium and boron during their reduction by a complex ferroalloy containing aluminum and silicon.
The paper presents the results of thermodynamic modeling of equilibrium interphase distribution of chromium and boron reduced by silicon and aluminum from the СаО – SiO2 – В2O3 – Al2O3 – Cr2O3 – MgO oxide system by aluminum ferrosilicon, a complex ferroalloy.
Materials and methods
We performed thermodynamic modeling of the equilibrium interphase distribution of chromium and boron reduced by silicon and aluminum of the complex reducing agent (aluminum ferrosilicon) from the СаО – SiO2 – В2O3 – Al2O3 – Cr2O3 – MgO oxide system using the HSC Chemistry 6.12 software package. This software is based on the calculating equilibrium compositions and the amount of resulting products, using the Gibbs energy minimization algorithm.
Thermodynamic modeling was performed for the temperatures of 1600 and 1700 °C. The mass of the working medium was 115 kg (100 kg of metal and 15 kg of slag) with the gas phase (N2 ) volume of 2.24 m3 and the system pressure of 0.098 MPa. The amount of the reducing agent is 0.89 kg. The interphase distribution coefficients of chromium and boron were obtained by their concentration ratios in the slag and metal (LB = (B2O3)/[B] and LCr = (Cr2O3 )/[Cr]).
The oxide system composition corresponds to 16 points of the local simplex plan and is presented in Table 1. In addition to calcium, silicon and boron oxides, all slags include chromium, magnesium and aluminum oxides in the amount of 12, 8 and 3 %, respectively. The metal part is represented by stainless steel containing, %: 16.0 Cr; 0.03 C; 0.28 Si; 0.010 S; 1.46 Mn; 6.98 Ni; 0.01 Al; the rest is Fe and aluminum ferrosilicon (AFS), a complex alloy containing, %: 55.8 Si; 18.8 Al; 25.4 Fe.
Table 1. Composition of slag in 16 points of the local simplex plan
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The results of thermodynamic modeling are presented by approximating mathematical models in the form of a reduced polynomial of the third degree, which describe the influence of the slag composition of the studied oxide system on the interphase distribution coefficients of chromium and boron at temperatures of 1600 and 1700 °C [18]. The adequacy of the constructed mathematical models was tested by three control points, not included in the experiment planning matrix, using t-criterion at the significance level of 0.01.
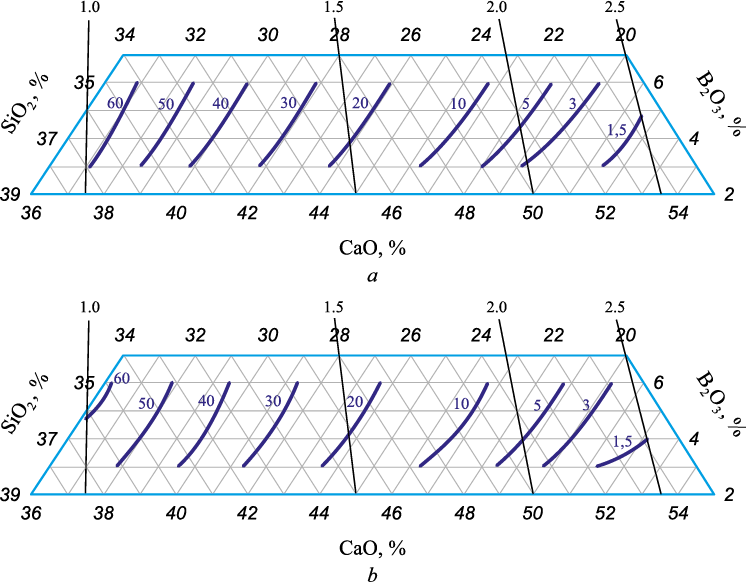
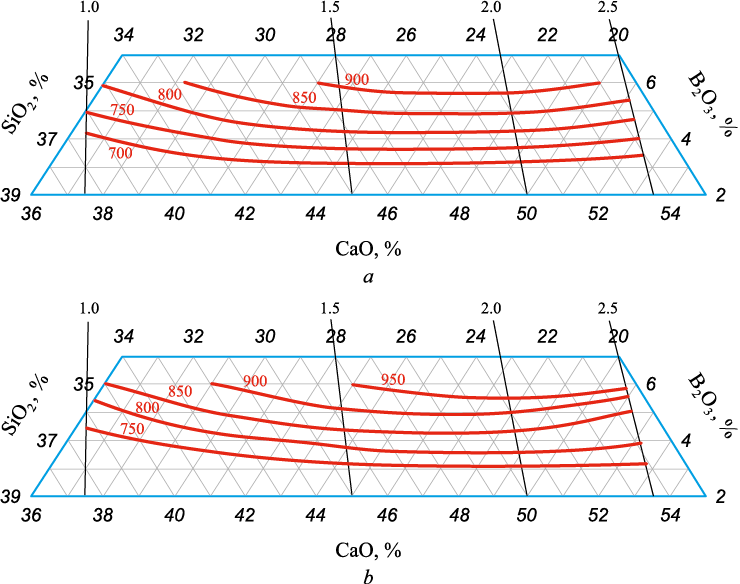
Figs. 1 and 2 graphically present the results of mathematical modeling in the form of composition – property diagrams. The solid lines show isolines of the equilibrium interphase distribution of chromium and boron, while thin lines reflect the basicity of the slag (CaO/SiO2) with its value indicated.
Fig. 1. Dependence of the coefficient of equilibrium interphase distribution of chromium
Fig. 2. Dependence of the coefficient of equilibrium interphase distribution of boron |
Along with thermodynamic modeling of the equilibrium interphase distribution of chromium and boron, we conducted high-temperature experimental studies using an electric resistance furnace in magnesia crucibles in an argon current at 1600 °C. The low-carbon stainless steel was held for 30 min under slag in the points used for local simplex. The temperature was measured using a BP5/20 tungsten thermocouple. Metal samples were prepared from chips of AISI 304 stainless steel and steel ST3SP, as well as the slag from two base points Y1 and Y3 (Table 1). We took the test charge of ground metal and slag in the quantities of 75 and 50 g to achieve the maximum phase contact surface and to exclude the influence of the mass of metal and slag phases on the interphase distribution coefficients of chromium and boron [19].
Results and discussion
Table 2 and Figs. 1, 2 present the results of thermodynamic modeling of the equilibrium interphase distribution of chromium and boron depending on the basicity of the slags of the studied oxide system and temperature.
Table 2. Interfacial distribution coefficient of chromium and boron
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
With the slag basicity ranging from 1.0 to 1.5 and the boron oxide concentration varying from 3 to 6 wt. %, the interphase distribution coefficient of chromium dropped from 60·10\(^–\)3 to 20·10\(^–\)3 at 1600 °C (Fig. 1, а). The increase in the slags basicity to 2.5, with the boron oxide concentration changing from 5 to 3 %, results in drop of the interphase distribution coefficient of chromium to 1.5·10\(^–\)3, which is indicative of more effective chromium reduction due to growing basicity of formed slags. The increase in boron oxide concentration comes with a slight deterioration of the chromium reduction process. The rise in the B2O3 content in the slag from 3 to 6 % is accompanied (for example, at basicity of 2.0) by an increase in the equilibrium interphase distribution coefficient of chromium from 3·10\(^–\)3 to 5·10\(^–\)3 at a temperature of 1600 °C (Fig. 1, а). The temperature rise from 1600 to 1700 °C has a slight impact on the interphase distribution coefficient of chromium (Table 2). At 1700 °C in the considered range of basicity and boron oxide content, it remains at the level ranging from 60·10\(^–\)3 to 1.5·10\(^–\)3 (Fig. 1, b).
With the basicity of formed slags ranging from 1.0 to 2.5, as the boron oxide concentration rises from 3 to 6 %, the equilibrium interphase distribution coefficient of boron increases from 700 to 900 (Fig. 2, а). We can clearly observe the influence of boron oxide on the equilibrium interphase distribution coefficient, while the impact of basicity is weak. For example, in the basicity range of 1.5 – 2.5, the equilibrium interphase distribution coefficient of boron is 900, its oxide concentration ranging from 5.7 to 6.0 %. The decrease of boron oxide concentration to 5.0 – 5.3 % in the considered basicity range causes the drop of the equilibrium interphase distribution coefficient of boron to 850. The behavior of the equilibrium interphase distribution coefficient of boron follows a similar pattern when the boron oxide concentration reaches 3.0 – 3.4 %.
The process temperature rising to 1700 °C leads to a slight increase in LB by 50 units and deteriorates the process of boron reduction (Fig. 2, b).
The positive impact of basicity of formed slags in the studied range of the chemical composition on the chromium and boron reduction can be qualitatively explained in terms of the phase composition formation (Table 3) and thermodynamics of reactions of chromium and boron reduction by aluminum and silicon (Table 4).
Table 3. Slag phases involved in reduction of chromium and boron at 1600 °C
Table 4. Change in Gibbs energy during reduction
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
According to the results of thermodynamic modeling (Table 3), the composition of chromium-containing phases of low-base slag Y1 is represented mainly by Cr2O3 and CaO·Cr2O3 phases, the number of which decreases in the process of chromium reduction from 8.1 and 5.4 % to 0.7 and 0.4 %, respectively. At the same time, the process of chromium reduction by silicon mainly occurs following the reactions (1) and (2) (Table 4), which is confirmed by the increase in content of SiO2 and CaSiO3 in the final slag from 4.8 and 18.8 % to 5.8 and 20.6 %, respectively (Table 3). The reduction of chromium by aluminum occurs following the reactions (6) and (7) (Table 4), which is confirmed by the increase in the content of the products of these reactions Al2O3 and CaO·Al2O3 from 0.8 and 0.3 % to 1.2 and 0.4 % (Table 3).
The reduction of chromium from low-base slag Y4 (basicity of 1.0) occurs following the same reactions as for slag Y1 . Low-base slags are characterized by reduction of chromium mainly by aluminum as a result of reactions (6) and (7) with partial development of silicothermic reactions (1) and (2) (Table 4), which is attributed to the higher negative ΔG value of aluminothermic reactions compared to silicothermic ones.
In the highly basic slag Y2 (basicity of 2.5) in the presence of a large amount of free CaO, the reduction of chromium by silicon is more active (reactions (2) – (4)) (Table 4). Due to the slag high basicity, the content of CaO·Cr2O3 in Y2 is much higher than in low-base Y1 . Therefore, a considerable amount of this phase is reduced by aluminum following the reaction (7) (Table 4). It should be noted that after chromium reduction, the chromium-containing phases are present in negligible amounts, i.е., the content of Cr2O3 drops from 1.0 to 0.002 % and that of CaO·Cr2O3 – from 15.1 to 0.02 % (Table 3). Similarly, chromium is reduced from the high-base slag Y3 .
Boron is insignificantly reduced from the slag as the change of Gibbs free energy of its reduction by aluminum and silicon from calcium borates is minimal, therefore, the transition of boron to metal for all studied slag compositions following the reactions (8) – (10) is insignificant (Table 4).
We conducted high-temperature experimental studies to verify if the results of thermodynamic modeling of the chromium and boron interphase distribution are adequate. The experiment showed that the Cr2O3 content in the slag at 1600 °C is 0.96 wt. %, which corresponds to the interphase distribution coefficient of 49.8·10\(^–\)3. The interphase distribution of boron reaches 648 with a residual B2O3 content in the slag of 3.89 wt. %. In general, the experimental results are close to the thermodynamic modeling, the kinetic factors accounting for the difference between them.
Conclusions
The thermodynamic modeling enabled us to obtain new data, based on which we constructed approximating mathematical models of the composition – property relation with graphical representation in the form of diagrams showing the equilibrium interphase distribution of chromium and boron depending on the process temperature, B2O3 content and basicity of the studied oxide system. Based on the plotted diagrams, we conducted quantitative evaluation of the impact that the above-mentioned factors had on the equilibrium interphase distribution of chromium and boron.
It was found that the increase in the basicity of the oxide system from 1.0 to 2.5, other conditions being equal, favorably affects the completeness of chromium reduction. At the same time, the increase in boron oxide concentration is accompanied by a slight decrease of the chromium reduction. The process temperature rise has no significant effect on the chromium reduction, but negatively affects the reduction of boron. It was determined that chromium is mostly reduced by aluminum with partial development of silicothermic reactions. The performed high-temperature experiment confirmed the results of thermodynamic modeling.
References
1. Illarionov A.G., Grib S.V., Yurovskikh A.S., Volokitina E.A., Gilev M.V., Azorina T.S. Usage of metal materials for medical implants. Bulletin of the Ivanovo Medical Academy. 2017;22(4):46–50. (In Russ.).
2. Bel’tyukov E.A., Shartdinov R.R., Lobanova L.A., etс. Effect of boron on the properties of stainless steels. In: Ural School of Young Metallurgists: materials of the XXI Int. Sci. and Tech. Ural School-Seminar of Metallurgists-Young Scientists, February 07–11, 2022. Yekaterinburg: UrFU; 2022:394–399. (In Russ.).
3. Capitan M.J., Lefebvre S., Traverse A., Paúl A., Odriozola J.A. Anomalous scattering study of oxide scales formed at 1173 K on surface modified stainless steel. Journal of Materials Chemistry. 1998;8(10):2293–2298. https://doi.org/10.1039/A802233J
4. Wei D.B., Huang J.X., Zhang A.W., Jiang Z.Y., Tieu A.K., Shi X., Jiao S.H., Qu X.Y. Study on the oxidation of stainless steels 304 and 304L in humid air and the friction during hot rolling. Wear. 2009;267(9-10):1741–1745. https://doi.org/10.1016/j.wear.2008.11.030
5. Gribkov A.A., Brodov A.A. Present and future of Russian market of stainless steel. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2021;77(2):193–199. (In Russ.). https://doi.org/10.32339/0135-5910-2021-2-193-199
6. Tokovoi O.K. Argon-Oxygen Decarburization of Stainless Steel: Monograph. Chelyabinsk: SUSU; 2015:250. (In Russ.).
7. Cai J., Li J. Decarburization and chromium conservation model in AOD refining process of 304 stainless steel. In: 12th Int. Symp. on High-Temperature Metallurgical Processing. The Minerals, Metals & Materials Series. Springer: Cham; 2022:71-80. https://doi.org/10.1007/978-3-030-92388-4_7
8. Povolotskii D.Ya., Gudim Yu.A. Production of Stainless Steel. Chelyabinsk: SUSU; 1988:236. (In Russ.).
9. Kornievskii V.N., Panchenko A.I., Logozinskii I.N., etс. Development of technology for ladle-furnace processing of electric steel using pegmatite as a substitute for fluorspar. Sovremennaya elektrometallurgiya. 2015;(4):38–46. (In Russ.).
10. Morii L., Kumura Sh., Mori H., Shinkai M., Sakuma H. Development of new refining process for manufacture of stainless steel. DENKI-SEIKO. 1993;64(1):4–12. https://doi.org/10.4262/denkiseiko.64.4
11. Hongming W., Tingwang Z., Hua Z. Effect of B2O3 on melting temperature, viscosity and desulfurization capacity of CaO-based refining flux. ISIJ International. 2011;51(5): 702–708. http://dx.doi.org/10.2355/isijinternational.51.702
12. Forsbacka L. Experimental study and modelling of viscosity of chromium containing slags: Dr. Tech. Sci. Diss, Helsinki; 2007:88.
13. Wu T., Zhang Y., Yuan F., An Z. Effects of the Cr2O3 content on the viscosity of CaO-SiO2-10 Pct Al2O3-Cr2O3 quaternary slag. Metallurgical and Materials Transactions B. 2018;49:1719–1731. https://doi.org/10.1007/s11663-018-1258-z
14. Akberdin A.A., Kireeva G.M., Kim A.S. Physical properties of boron-containing blast furnace slags. Kompleksnoe ispol’zovanie mineral’nogo syr’ya. 1996;(3):27–31. (In Russ.).
15. Babenko A.A., Zhuchkov V.I., Upolovnikova A.G., Kel’ I.N. Study of the viscosity of slags of СаО – SiO2 – В2О3 – 25 % Al2O3 – 8 % MgO system. Izvestiya. Ferrous Metallurgy. 2017;60(10):820–825. (In Russ.). https://doi.org/10.17073/0368-0797-2017-10-820-825
16. Mills K.C., Yuan L., Li Z., Zhang G.H., Chou K.C. A review of the factors affecting the thermophysical properties of silicate slags. High Temperature Materials and Processes. 2012;31(4–5):301–321. https://doi.org/10.1515/htmp-2012-0097
17. Nazyuta L.Yu., Tikhonyuk L.S., Kostyrya I.N., etс. Features of microalloying with boron in the smelting of low-alloy structural steels. Metall i lit’e Ukrainy. 2018;298–299(3–4): 18–27. (In Russ.).
18. Kim V.A., Nikolai EH.N., Akberdin A.A., etс. Planning an Experiment when Studying the Physical and Chemical Properties of Metallurgical Slags: A Methodological Manual. Alma-Ata: Nauka; 1989:116. (In Russ.).
19. Boronenkov V.N., Zinigrad M.I., Leont’ev L.I., etс. Modeling of the Structure, Properties and Processes of Interphase Interaction in the Metal-Oxide Melt-Gas System. Yekaterinburg: UB RAS; 2010:452. (In Russ.).
About the Authors
A. A. BabenkoRussian Federation
Anatoly A. Babenko, Dr. Sci. (Eng.), Prof., Chief Researcher, Head of the Department of Ferrous Metallurgy
101 Amundsen Str., Yekaterinburg 620016, Russian Federation
V. I. Zhuchkov
Russian Federation
Vladimir I. Zhuchkov, Dr. Sci. (Eng.), Prof., Chief Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsen Str., Yekaterinburg 620016, Russian Federation
N. I. Kel’
Russian Federation
Il’ya N. Kel’, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsen Str., Yekaterinburg 620016, Russian Federation
A. G. Upolovnikova
Russian Federation
Alena G. Upolovnikova, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsen Str., Yekaterinburg 620016, Russian Federation
R. R. Shartdinov
Russian Federation
Ruslan R. Shartdinov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsen Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Babenko A.A., Zhuchkov V.I., Kel’ N.I., Upolovnikova A.G., Shartdinov R.R. Thermodynamic modeling of interphase distribution of chromium and boron in slags of AOD reduction period. Izvestiya. Ferrous Metallurgy. 2024;67(3):351-359. https://doi.org/10.17073/0368-0797-2024-3-351-359