Scroll to:
Effect of basicity on physical properties of ladle slags of CaO ‒ SiO2 ‒ Ce2O3 ‒ Al2O3 ‒ MgO system
https://doi.org/10.17073/0368-0797-2024-2-205-210
Abstract
The authors studied the physical properties of the slags of CaO ‒ SiO2 ‒ Al2O3 ‒ MgO system containing cerium oxide. The developed slags are based on a calcium silicate system, the basicity (CaO)/(SiO2) of which has a great influence on the slag properties. Generalization of the performed studies results allowed obtaining new data on the effect of basicity in cerium-containing slags of the studied oxide system on viscosity, temperature of crystallization onset and structure. Experimental studies of the physical properties of cerium-containing slags showed that with an increase in basicity of 2.0 ‒ 5.0, an increase in temperature of crystallization onset and viscosity is observed associated with structure of the formed slags. An increase in basicity from 2.0 to 5.0 contributes to an increase in viscosity from 0.20 to 0.41 Pa·s at 1500 °C and an increase in the crystallization temperature from 1397 to 1497 °C. The structural analysis showed that the structure of the cerium-containing slag is influenced by both the Si4+ ion and the Al3+ ion, which are grid-forming agents. Silicon ions in this system are present in the form of [SiO4 ]-tetrahedra, whereas aluminum ions are present in form of [AlO4]-tetrahedra and [AlO6]-octahedra. With an increase in basicity 2.0 to 2.5, the silicon structure becomes more complicated, and then at a basicity of 3.5 ‒ 5.0 it becomes simpler, whereas the aluminate one becomes more complicated due to an increase in the content of CaO, which participates in charge compensation of polymerized structural units [AlO4 ]-tetrahedra with the formation of a more stable tetrahedral structure, and as a result of increased slag viscosity. Slags of the studied oxide system containing 15 % Ce2O3 are characterized by a sufficiently high liquid mobility in the considered basicity range.
Keywords
For citations:
Upolovnikova A.G., Shartdinov R.R., Smetannikov A.N. Effect of basicity on physical properties of ladle slags of CaO ‒ SiO2 ‒ Ce2O3 ‒ Al2O3 ‒ MgO system. Izvestiya. Ferrous Metallurgy. 2024;67(2):205-210. https://doi.org/10.17073/0368-0797-2024-2-205-210
Introduction
Viscosity stands out as one of the most crucial physical properties of slag, as metallurgical processes hinge on phenomena influenced by heat and mass transfer within both slag and metal [1; 2]. Exploring the utilization of rare earth element (REE) oxides represents a promising avenue for decreasing the viscosity of refining slags. Investigations into the impact of cerium oxide additives on slag’s physical properties have revealed that cerium oxide reduces both viscosity and crystallization temperature [3 ‒ 5]. Recent studies have demonstrated that incorporating rare earth oxides into slag can diminish the activity of Al2O3 oxide in the slag while enhancing the slag’s adsorption capacity for Al2O3 inclusions in the metal [6 ‒ 8]. Additionally, the equilibrium between refining slag containing Ce2O3 and molten steel deoxidized with aluminum hints at the potential for introducing a small amount of cerium, which may transfer into the steel [9 – 11], thereby facilitating micro-alloying and modification [12]. However, to date, the influence of basicity on the physical properties of cerium-containing ladle slags remains unexplored in both domestic and foreign literature.
This study aimed to investigate the physical properties of slags within the СаО ‒ SiO2 ‒ Ce2O3 ‒ Al2O3 ‒ MgO system. By consolidating research findings, we sought to generate new insights into the impact of basicity on the viscosity, temperature of crystallization onset, and structure of cerium-containing slags within the studied oxide system.
Research methods
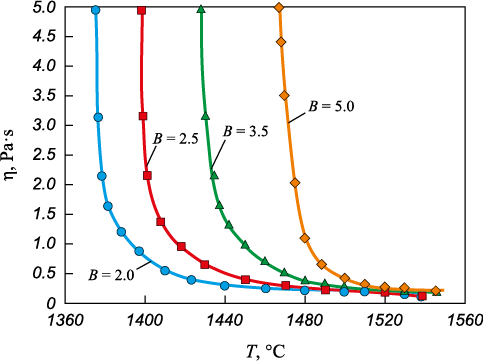
The slags of the СаО ‒ SiO2 ‒ Ce2O3 ‒ Al2O3 ‒ MgO oxide system were melted in a resistance furnace using graphite crucibles under an argon atmosphere. Analytical grade oxides were calcined for 2 – 3 h at a temperature of 800 °C prior to melting. Viscosity measurements of the slags were conducted in graphite crucibles using an electric vibrating viscometer, with continuous cooling of the melt from a homogeneous-liquid to a solid state [13]. A molybdenum rod with a diameter of 1.5 mm served as the measuring spindle. The temperature of the slag was monitored using a VR 5/20 tungsten-rhenium thermocouple. The crystallization temperature of the slags was determined according to Frenkel’s theory of viscous flow. For this purpose, graphs were plotted in coordinates ln η – 1/T, with breaks indicating the onset temperature of slag crystallization [14]. The results of viscosity and crystallization temperature measurements are presented in Table 1 and Fig. 1.
Table 1. Composition, temperature of crystallization onset
Fig. 1. Dependence of viscosity of the slags on temperature | ||||||||||||||||||||||||||||||||||||||||||||||||||||
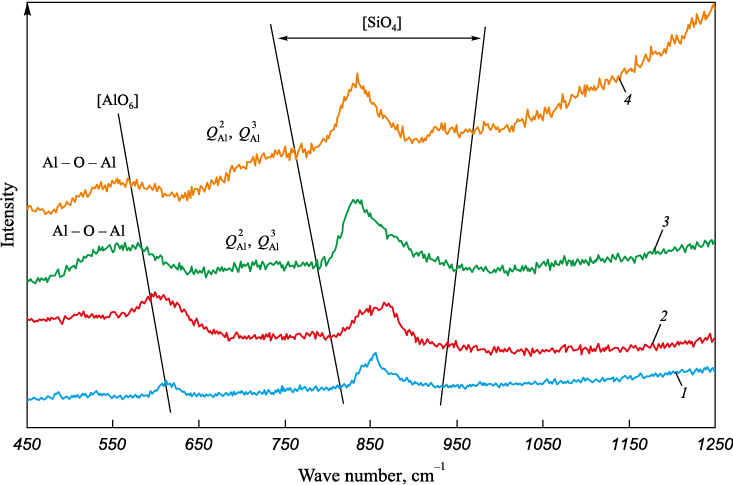
The structure of test slag samples was investigated using a Raman microscope spectrometer U 1000 with a laser having an excitation wavelength of 532 nm. The obtained spectra, within the wave number range of 450 – 1250 cm\(^‒\)1, are depicted in Fig. 2. The observed lines in such spectra can be clearly attributed to vibrations of the molecules of the material under study. Depending on the frequency, intensity, and shape of these lines, conclusions about the structure of the slags can be drawn [15]. This transformation can be attributed to the characteristics of the slag structure. Fig. 2 displays the Raman spectra of slag samples with varying basicity, featuring peaks: in the low-frequency region with wave numbers around 600 cm\(^‒\)1, representing Al ‒ O stretching vibrations in [AlO6 ] octahedra within the range of slag basicity from 2.0 to 2.5; as basicity increases to 3.5 – 5.0 units, peaks emerge in the region of about 550 cm\(^‒\)1, attributed to the transverse motion of bridging oxygen inside the Al ‒ O ‒ Al bond; peaks in the range of 650 ‒ 800 cm\(^‒\)1, reflecting Al – O stretching vibrations in [AlO4] tetrahedra. Peaks in the higher wave numbers region (800 – 950 cm\(^‒\)1) are associated with the silicate structure ([SiO4]-tetrahedra). The intensity and shape of these peaks allow for the evaluation of the influence of basicity on the structure of the formed slags and their viscosity.
Fig. 2. Raman spectra of the slags at basicity of 2.0 (1), 2.5 (2), 3.5 (3) and 5.0 (4) |
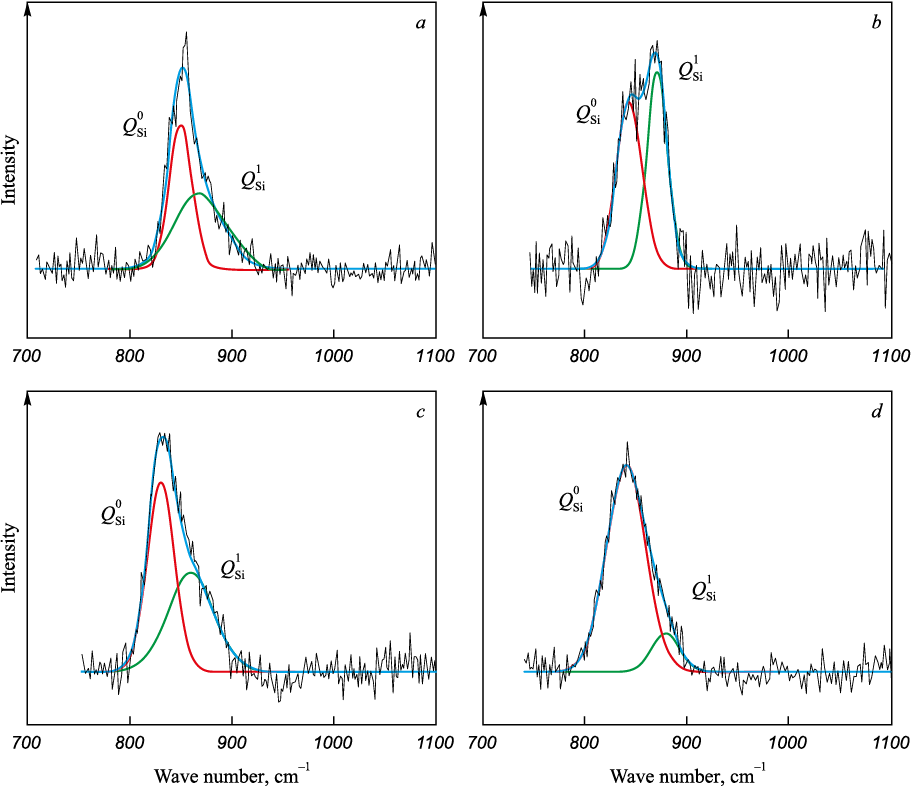
For further quantitative determination of changes in structural units with different basicity of the slag, the Raman spectra (Fig. 2) were deconvoluted by the Gaussian method using the PeakFit software with the correlation coefficient of minimum 0.99. The results of deconvolution of the silicate region of Raman spectra are shown in Fig. 3 and Table 2.
Fig. 3. Deconvolution of the silicate region at basicity of 2.0 (а), 2.5 (b), 3.5 (c) and 5.0 (d)
Table 2. Proportion of structural elements
|
Results and discussion
The viscosity dependence of the studied slags on temperature, within a basicity range of 2.0 to 5.0, is illustrated in Fig. 1. As basicity increases, slags transition gradually from fluid states with low crystallization temperatures to those with higher viscosity and crystallization onset temperatures (Table 1).
The slag structure formed at a basicity of 2.0 is low-polymerized. As noted earlier, it is characterized by the presence of [AlO6]-octahedra, which function as grid modifier (Fig. 2), along with two depolymerized structural units of silicon: [SiO4]4\(^–\) with an increased proportion of non-bridging oxygen to 0.48 (\(Q_{{\rm{Si}}}^0\)) and [Si2O7]6\(^–\) with a proportion of one bridging oxygen increased to 0.52 (\(Q_{{\rm{Si}}}^1\)) Fig. 3, Table 2). This structure arises due to the presence of structure modifiers – calcium and cerium oxides – in the slag. Their dissociation in melts releases additional O2\(^–\) ions, which interact with [AlO4 ]- and [SiO4 ]-tetrahedra, disrupting the aluminate and silicate melt structures [16; 17]. Consequently, these slags exhibit a low crystallization temperature (1397 °C) and low viscosity (0.20 and 0.16 Pa·s) at temperatures of 1500 and 1550 °C (Table 1).
As the basicity increases to 2.5, the silicate structure becomes more complex. Its polymerization degree rises from 0.52 to 0.59, while the proportion of non-bridging oxygen decreases from 0.48 to 0.41, and the proportion with one bridging oxygen increases from 0.52 to 0.59 (Table 2). In high-basicity slags containing Al2O3 , Al3+ ions are absorbed by the silicate structure, acting as grid-forming agents, thus enhancing the complexity of the silicate structure [18]. The increased complexity of the slag structure elevates the onset temperature of crystallization to 1419 °C and viscosity to 0.22 and 0.17 Pa·s at temperatures of 1500 and 1550 °C (Table 1).
As the basicity increases to 3.5 and 5.0, a peak emerges in the region of about 550 cm\(^–\)1, attributed to the transverse motion of bridging oxygen inside the Al – O – Al bond. The relative Al – О – Al intensity gradually increases with increasing basicity, while the opposite trend is observed for [AlO6] octahedra. This indicates an enhanced Al – O – Al bond and a decreased proportion of [AlO6 ] octahedra, leading to polymerization of the aluminate grid. Additionally, an increase in the slag basicity from 3.5 to 5.0 results in higher peak intensity in the region of wave numbers 650 – 800 cm\(^–\)1. This is attributed to symmetrical stretching vibrations [AlO3]3\(^–\) (\(Q_{{\rm{Al}}}^2\)) and [Al2O5]4\(^–\) (\(Q_{{\rm{Al}}}^3\)) [16; 17], indicating that the aluminate structure in the molten slag (Fig. 2) has become more complex, resulting in an increase in the crystallization onset temperature to 1463 and 1497 °C. Viscosity increases to 0.26 and 0.18 Pa·s at temperatures of 1500 and 1550 °C and a basicity of 3.50 and to 0.41 and 0.23 Pa·s at temperatures of 1500 and 1550 °C and a basicity of 5.0.
Alongside the polymerization of the aluminate structure at basicities of 3.5 and 5.0, the silicate structure becomes simpler, and the polymerization degree decreases from 0.47 to 0.12 (Table 2). CaO oxide can act not only as a grid modifier but also as a charge compensator due to the excess of Ca2+ ions formed with increasing basicity. Са2+ cations will compensate for the polymerized structural units of [AlO4]-tetrahedra, leading to the formation of a more stable tetrahedral structure and resulting in increased slag viscosity [19; 20]. Cerium ions can act as charge compensators and stabilize the aluminate structure [3; 21; 22].
Conclusions
Experimental studies of the physical properties of the slags within the СаО ‒ SiO2 ‒ Ce2O3 ‒ Al2O3 ‒ MgO oxide system have indicated that increasing the basicity from 2.0 to 5.0 leads to higher viscosity and temperature of crystallization onset. This phenomenon is attributed to the structure of the resulting slags. With the augmentation of basicity, the aluminate structure becomes more complex, while the silicate structure becomes simpler due to an excess of Ca2+ ions, which serve as charge compensators for the polymerized structural units of [AlO4] tetrahedra. Overall, the slags within the studied oxide system containing 15 % Ce2О3 exhibit sufficiently high fluidity within the considered basicity range.
References
1. Popel’ S.I. Theory of Metallurgical Processes. Moscow: Metallurgiya; 1986:463. (In Russ.).
2. Sokolov G.A. Extra-Furnace Refining of Steel. Moscow: Metallurgiya; 1977:208. (In Russ.).
3. Wu C., Cheng G., Long H. Effect of Ce2O3 and CaO/Al2O3 on the phase, melting temperature and viscosity of CaO ‒ Al2O3 ‒ 10 mass % SiO2 based slags. High Temperature Materials and Processes. 2014;33(1):77–84. http://dx.doi.org/10.1515/htmp-2013-0025
4. Liu C., Qi J., Sun J., Zhang X. Design and fluidity research of a new tundish flux for rare earth steel. Journal of Sustainable Metallurgy. 2022;8:1104–1116. https://doi.org/10.1007/s40831-022-00544-6
5. Zheng X., Liu C. Effect of Ce2O3 on the melt structure and properties of CaO – Al2O3-based slag. ISIJ International. 2022;62(6):1091–1098. https://doi.org/10.2355/isijinternational.ISIJINT-2021-545
6. Wang L.J., Wang Q., Li J.M., Chou K.C. Dissolution mechanism of Al2O3 in refining slags containing Ce2O3 . Journal of Mining and Metallurgy, Section B: Metallurgy. 2016;52(1): 35‒40. https://doi.org/10.2298/JMMB140706004W
7. Liu Y.Q., Wang L.J., Chou K.C. Dissolution behavior of Al2O3 in refining slags containing Ce2O3. ISIJ International. 2014;54(4):728–733. http://dx.doi.org/10.2355/isijinternational.54.728
8. Cao J., Li Y., Lin W., Che J., Zhou F., Tan Y., Li D., Chen C., Dang J. Assessment of inclusion removal ability in refining slags containing Ce2O3. Crystals. 2023;13(2):202. https://doi.org/10.3390/cryst13020202
9. Yang X., Long H., Cheng G., Wu C., Wu B. Effect of refining slag containing Ce2O3 on steel cleanliness. Journal of Rare Earths. 2011;29(11):1079–1083. https://doi.org/10.1016/S1002-0721(10)60602-3
10. Babenko A.A., Smirnov L.A., Upolovnikova A.G., Shartdinov R.R. Study of possibility of cerium reduction from slags of CaO–SiO2–Ce2O3–15%Al2O3–8%MgO system. In: IOP Conference Series: Materials Science and Engineering. 2020;966:012010. https://doi.org/10.1088/1757-899X/966/1/012010
11. Upolovnikova A.G., Babenko A.A., Smirnov L.A., Mikhailova L.Yu. Direct microalloying of steel with cerium under slags of СаО – SiO2 – Ce2O3 – 15 % Al2O3 – 8 % MgO system with additional reducing agents. Izvestiya. Ferrous Metallurgy. 2021;64(8):581–587. (In Russ.). https://doi.org/10.17073/0368-0797-2021-8-581-587
12. Kudrin V.A. Extra-Furnace Treatment of Cast Iron and Steel. Moscow: Metallurgiya;1992:336. (In Russ.).
13. Shtengel’meier S.V., Prusov V.A., Bogachev V.A. Improvement of the viscosity measurement technique with a vibrating viscometer. Zavodskaya laboratoriya. 1985;51(9):56–57. (In Russ.).
14. Voskoboinikov V.G., Dunaev N.E., Mikhalevich A.G., Kukhtin T.I., Shtengel’meier S.V. Properties of Liquid Blast Furnace Slags. Moscow: Metallurgiya;1975:180.
15. Böcker J. Spektroskopie. Instrumentelle Analytik mit Atom- und Molekülspektrometrie. Würzburg: Vogel Buchverlag; 1997:519. (In Germ.).
16. Zhang R., Wang Z., Meng Y., Jiao S., Jia J., Min Y., Liu C. Quantitative insight into aluminum structures in CaO–Al2O3–SiO2 system via Raman and 27Al MAS-NMR spectroscopies. Journal of Non-Crystalline Solids. 2021;573:121116. https://doi.org/10.1016/j.jnoncrysol.2021.121116
17. Kim T.S., Park J.H. Structure-viscosity relationship of low-silica calcium aluminsilicate melts. ISIJ International. 2014;54(9):2031–2038. https://doi.org/10.2355/isijinternational.54.2031
18. Gao J., Wen G., Huang T., Tang P., Liu Q. Effects of the composition on the structure and viscosity of the CaO–SiO2-based mold flux. Journal of Non-Crystalline Solids. 2016; 435:33–39. https://doi.org/10.1016/j.jnoncrysol.2016.01.001
19. Zheng D.-L., Ma G.-J., Zhang X., Liu M.-K., Xu J. Effect of CaO/Al2O3 on structure, viscosity, and surface tension of electroslag remelting-type CeO2-bearing slag. Journal of Iron and Steel Research International. 2023;30:717–725. https://doi.org/10.1007/s42243-022-00844-x
20. Qi J., Liu C., Zhang C., Jiang M. Effect of Ce2O3 on structure, viscosity, and crystalline phase of CaO–Al2O3–Li2O–Ce2O3. Metallurgical and Materials Transactions B. 2017;48:11–16. https://doi.org/10.1007/s11663-016-0850-3
21. Zheng X., Liu C. Investigation of CaO/Al2O3 mass ratio on the properties and structure of Ce2O3-containing CaO–Al2O3-based tundish flux. ISIJ International. 2022;62(3):418–425. https://doi.org/10.2355/isijinternational.ISIJINT-2021-438
22. Lin S.-L., Hwang C.-S. Structures of CeO2–A12O3–SiO2 glasses. Japanese Journal of Applied Physics. 1996;35(7R): 3975. https://doi.org/10.1143/JJAP.35.3975
About the Authors
A. G. UpolovnikovaRussian Federation
Alena G. Upolovnikova, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
R. R. Shartdinov
Russian Federation
Ruslan R. Shartdinov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. N. Smetannikov
Russian Federation
Artem N. Smetannikov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Upolovnikova A.G., Shartdinov R.R., Smetannikov A.N. Effect of basicity on physical properties of ladle slags of CaO ‒ SiO2 ‒ Ce2O3 ‒ Al2O3 ‒ MgO system. Izvestiya. Ferrous Metallurgy. 2024;67(2):205-210. https://doi.org/10.17073/0368-0797-2024-2-205-210