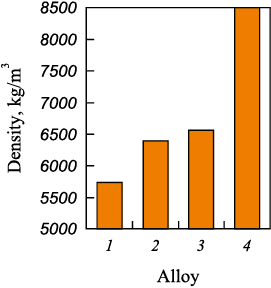Scroll to:
Physicochemical characteristics of new complex niobium-containing alloys
https://doi.org/10.17073/0368-0797-2023-5-616-622
Abstract
The authors studied the physicochemical characteristics of new complex alloys containing, %: 11 – 30 Nb, 23 – 28 Si, 3 – 10 Al and 3 – 4 Ti. It was shown that complex alloys have the most favorable values of density and crystallization temperatures compared to standard ferroniobium (60 wt. % Nb). Complex alloys with a low concentration of niobium have acceptable crystallization temperatures and optimal density values (5740 – 6560 kg/m3). This allows the pieces of ferroalloy to be completely in the liquid steel when it is released into the ladle, and to be constantly in motion, which increases absorption of the leading components. When the niobium concentration increases to 30 %, phase composition of the alloy changes: a decrease in the proportion of the low-temperature FeSi phase with low density values and an increase in the proportion of the high-density ternary compound NbFeSi2 with a crystallization temperature of ~1713 °C. An increase in Nb concentration from 11 to 17 % leads to a decrease in the crystallization temperature, and a further increase to 30 % Nb, on the contrary, is accompanied by an increase in the liquidus and solidus temperatures to 1700 and 1610 °C, respectively, which is consistent with liquidus line in phase diagram of the Fe – Nb system with a minimum in Nb concentration range ~18 %. The best characteristics, both from the point of view of obtaining ferroalloys and use for alloying steel, belong to an alloy containing, wt. %: 17.1 Nb, 24.6 Si, 7.6 Al and 3 Ti. This alloy is characterized by the temperature of crystallization onset (1550 °C) below the liquid steel bath temperature and belongs to the category of low-melting alloys. It has optimal density values – 6390 kg/m3, which has a positive effect on the performance characteristics of niobium ferroalloys.
Keywords
For citations:
Zayakin O.V., Kel’ I.N., Renev D.S., Sychev A.V., Mikhailova L.Yu., Dolmatov A.V. Physicochemical characteristics of new complex niobium-containing alloys. Izvestiya. Ferrous Metallurgy. 2023;66(5):616-622. https://doi.org/10.17073/0368-0797-2023-5-616-622
Introduction
In recent decades, there has been a significant increase in niobium consumption in Russia and globally. While previously primarily used to enhance the corrosion resistance of heat-resistant and stainless steels, niobium is now also employed to impart a strengthening effect in numerous grades of structural steel [1; 2].
Niobium, as an element, plays a crucial role in suppressing the recrystallization of austenite [3; 4]. The development of niobium carbonitrides Nb(C, N) near the grain boundaries of austenite leads to localized reduction in carbon concentration. The presence of the Nb(C, N) phase along the austenite grain boundaries serves as heterogeneous sites for initiating ferrite nucleation, thereby enhancing the metal’s plastic properties. Simultaneously, dissolved niobium exists in a liquid state within the steel, resulting in a dual effect. It aids in refining grain size by impeding the growth of austenitic grains during austenitization, achieved through segregation at the grain boundary and a reduction in their energy. Consequently, this decelerates the grain-boundary transition of ferrite while promoting martensitic or bainitic transformations [4; 5]. The coexistence of both ferrite and bainite contributes to enhancements in strength, ductility, and toughness. Consequently, altering the niobium type in steel enables the modification of resulting properties.
Given the mechanism through which niobium influences steel properties, its primary application lies in structural grades of steel for various purposes such as large-diameter gas and oil pipelines, shipbuilding, transportation, etc. [4; 6 – 10].
It’s important to highlight that within the global niobium consumption structure, the majority (over 88 %) is allocated for the production of high-strength low-alloy steels containing fractional percentages of niobium. The principal spectrum of niobium alloys consists primarily of various grades of ferroniobium, containing 55 – 70 wt. % Nb, manufactured through an aluminothermic process using pure niobium pentoxide or pyrochlore concentrate. Ferroalloys produced in Russian facilities typically contain, wt. %: 55 – 70 Nb; up to 6 Si; up to 8 Ti; up to 6 Al; up to 0.5 C; up to 0.3 S; up to 2 P; 1 – 8 Ta; the remaining component is Fe (State Standard GOST 16773 – 2003). The high concentration of niobium in the ferroalloy results in a higher melting point compared to the temperature of the molten metal (steel) being processed. This characteristic, in conjunction with the high density of the ferroalloy (~8500 kg/m3), causes solid pieces of ferroniobium to settle at the bottom of the ladle, subsequently dissolving at a slower rate. Consequently, this significantly extends the steel’s doping time and leads to an uneven distribution of niobium within the liquid metal volume [11].
Therefore, it is rational to explore the development of new complex niobium alloys that could offer more advantageous values for key parameters, including crystallization (melting) temperature and density [12].
The crystallization temperature (Tcr ) plays a pivotal role in both the production technology of alloys and their operational characteristics. However, the precise definition of this term often varies among publications, leading to conflicting information. Some sources present Tcr as a specific value [13], while others express it as a temperature range [14]. In the case of binary and ternary compounds, Tcr can be derived from their phase diagrams. However, for multicomponent systems containing complex alloys, determining the crystallization temperature typically necessitates experimental investigation.
Multicomponent alloys typically exhibit a range of temperatures within which they melt. Consequently, the alloy’s properties are more accurately characterized by the temperature at which crystallization initiates, known as the liquidus temperature Tl . However, discrepancies exist in publications regarding the optimal values for Tl [15 – 17]. According to the findings presented in [15], the melting temperature of ferroalloys should ideally fall within the range of 1100 – 1300 °C. Lower values might lead to oxidation of alloy components, necessitating the use of refractory materials to extend their melting duration. This paper proposes a conditional relative categorization of alloys into distinct groups based on temperature ranges: low-melting alloys (Tl < Tcr ), refractory alloys (Tcr < Tl < Tst. bath. ) and ultra-refractory alloys (Tl > Tst. bath. ), where Tst. bath. is the temperature of the steel bath.
Density (ρ) is an important technological property that significantly influences the degree and stability of assimilation of ferroalloy elements, the rate of their dissolution, and the uniformity of their distribution within the metal. This value is determined by the crystal structure and atomic mass of the elements comprising the alloy.
Density plays a crucial role in both the production and utilization of alloys. In ferroalloy production, it’s essential that the densities of the metal and slag differ significantly. When their densities are too close, it results in increased metal losses due to inadequate separation of metal from slag. This complication significantly impacts the technology involved in ferroalloy production.
The determination of density in ferroalloys can be accomplished through both experimental methods and calculations. Rational density values for ferroalloys were established via simulation in a laboratory unit and through calculation methodologies [11]. Ferroalloys are typically categorized as heavy (ρ > 7000 kg/m3), optimum (ρ = 5000 – 7000 kg/m3), or light (ρ < 5000 kg/m3). When introducing light ferroalloys into steel, they tend to become entrapped in slag and undergo partial oxidation. Conversely, heavy alloys settle at the bottom of ladles or melting units, slowly dissolving over time [18]. Alloys within the density range of 5000 – 7000 kg/m3 either remain completely submerged in liquid steel or create a small open area above the surface (no more than 10 %). Such positioning allows these alloys to be in motion and avoids oxidation by atmospheric oxygen, aiding in better absorption. Typically, ferroalloys are introduced into steel in solid form during the metal’s release from the furnace. The energy of the jet assists in mixing and immersing pieces of ferroalloy into the melt. Ferroalloys with optimum density are drawn into circulating bath streams, ensuring uniform distribution throughout the steel volume. This facilitates the complete and rapid dissolution of the ferroalloys.
Insufficient data regarding the properties of niobium ferroalloys are available in publications [19]. To develop rational compositions for new complex niobium-containing ferroalloys, the densities and crystallization temperatures of alloys within the Fe – Si – Al – Nb – Ti system were determined.
Materials and methods
The first stage of the experimentation involved obtaining experimental samples of complex alloys in laboratory settings using the method of melting in corundum crucibles within an argon flow, and these samples are outlined in Table 1.
Table 1. Chemical composition of niobium-containing alloys, wt. %*
| |||||||||||||||||||||||||||||||||
Among the alloys presented in Table 1, alloy 4 was selected as the reference sample. This particular alloy, corresponding to the niobium content similar to FNb60 grade ferroniobium, was chosen due to its widespread use in modern steelmaking practices.
To determine the crystallization temperatures, both the liquidus (Тl ) and solidus (Тs ) temperature curves were recorded during the cooling process of the alloys For this purpose, the samples were positioned within corundum crucibles placed in the operational zone of an electric resistance furnace. Temperature measurements were conducted using tungsten-rhenium thermocouples, specifically VR-5/20 with alundum tips, utilizing a Termodat-19M4 multimeter. During the measurements, one thermocouple’s tip was placed at the center of the melt, while the other was positioned in the working space of the furnace in close proximity to the crucible containing the melt. The temperature of the melt was determined based on the readings of the first thermocouple, while the furnace temperature was determined using the second thermocouple.
The samples were heated to temperatures ranging from 50 – 100 °C above the anticipated crystallization initiation temperature. Subsequently, they were cooled at a controlled rate of 10 – 15 °C/min, and temperature plateaus were recorded on the cooling curves. The first region observed on the cooling curves corresponded to the Тl , while the second region corresponded to the Тs .
The density of solid ferroalloys was measured using the pycnometric method, which has sufficient accuracy and ease of experimentation, in accordance with State Standard GOST 22524–77 [20].
The chemical composition of the samples was determined using inductively coupled plasma atomic emission spectrometry. The phase composition of the samples was identified through X-ray phase analysis using a Shimadzu XRD 7000C diffractometer (Ural-M Center for Shared Use).
Results and discussion
The findings concerning the physicochemical characteristics of the investigated niobium-containing alloys are detailed in Table 2. It is evident that all the complex niobium alloys under study exhibit more favorable values in terms of density and crystallization temperatures compared to high-percentage ferroniobium containing 60 % Nb.
Table 2. Physicochemical characteristics of niobium-containing alloys
| |||||||||||||||||||||||||||
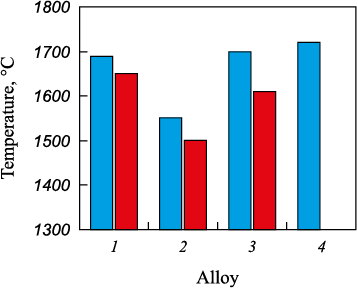
Figs. 1 and 2 depict the relationships showcasing changes in density and crystallization temperatures of complex alloys in relation to the niobium content.
Fig. 1. Dependence of complex alloy density
Fig. 2. Dependence of complex alloy crystallization temperatures |
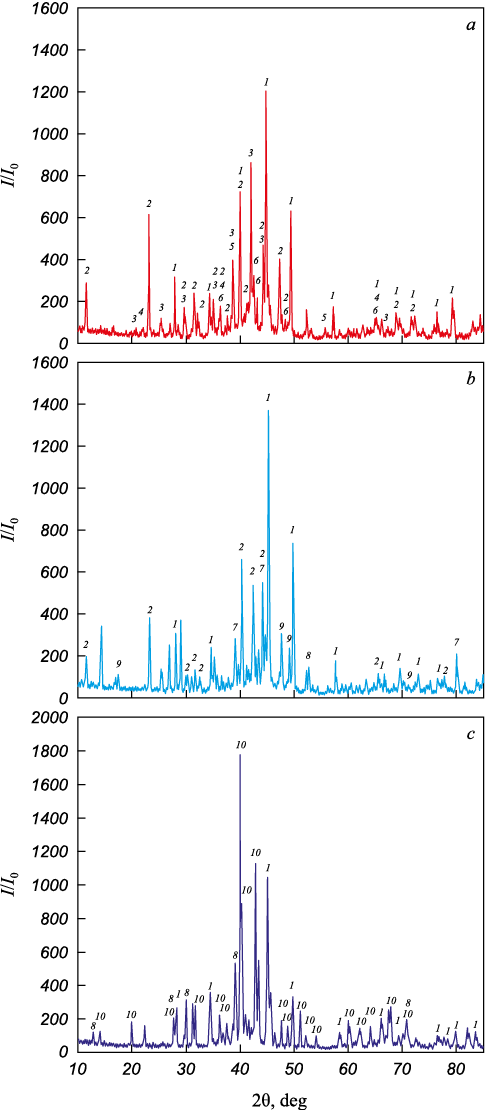
A reduction in the niobium fraction within complex alloys from 30 to 11.3 wt. % demonstrates a positive impact on their density, notably decreasing from 6560 to 5740 kg/m3. This is primarily attributed to niobium being the densest component of the alloy, with a density of 8570 kg/m3). Additionally, alterations in the phase composition (Fig. 3) also contribute to this observed phenomenon.
Fig. 3. XRD pattern of complex niobium-containing alloys: |
In alloy 1, a significant proportion of the FeSi phase (up to 63 %) contributes to reducing the overall density of the alloy. Conversely, in alloy 2, the formation of a ternary compound NbFeSi2 occurs, characterized by a high density of 6669 kg/m3. Additionally, there’s the presence of niobium intermetallic compound AlNb7 , which holds a density of 8431 kg/m3. As the niobium concentration escalates to 30 %, there’s a reduction in the fraction of the lighter FeSi phase and a subsequent increase in the fraction of the high-density ternary compound NbFeSi2 .
A comparable examination was conducted to investigate the impact of niobium concentration on the crystallization temperatures of complex alloys (Fig. 2).
Alloy 1 demonstrates a crystallization range spanning 1690 – 1650 °C, primarily due to the substantial presence of the FeSi phase (~60 %) with a crystallization temperature of 1550 °C. Elevating the niobium concentration from 11.3 to 17.1 % results in a decrease in the crystallization temperature, aligning with the liquidus line on the phase diagram of the Fe–Nb binary system as niobium concentration approaches ~18 % [21; 22]. A further elevation of niobium content in complex alloys to 30 % corresponds to an elevation in both the liquidus and solidus temperatures, reaching 1700 and 1610 °C, respectively. This trend mirrors the liquidus line observed in the Fe–Nb phase diagram, showing a peak at 1627 °C at ~45 % Nb. The notably high liquidus temperature values in alloy 3 are attributed to the considerable content (~53 %) of the refractory phase NbFeSi2 , initiating crystallization at ~1713 °С.
Overall, the findings regarding the correlation between niobium concentration and crystallization temperatures in complex alloys align qualitatively with existing data for binary alloys. Shifting from conventional high-percentage ferroniobium featuring 60 % Nb to complex alloys integrating silicon, aluminum, and reduced niobium concentrations allows the transition of alloys from super-refractory to refractory. Notably, alloy 2, comprising 17.1 % Nb, 24.6 % Si, 7.6 % Al and 3 % Ti, represents low-melting alloys, thereby positively influencing the performance characteristics of niobium ferroalloys.
Conclusions
The research into the physicochemical properties of novel complex niobium-based alloys has revealed promising attributes. These alloys, incorporating silicon, aluminum, and reduced niobium content, exhibit notably advantageous characteristics in terms of density and crystallization temperature when compared to the standard ferroniobium alloy (60 % Nb). Among these new alloys, the one containing 17.1 % Nb, 24.6 % Si, 7.6 % Al and 3 % Ti stands out for its exceptional qualities. With a crystallization initiation temperature of 1550 °C (lower than the liquid steel bath temperature), this alloy falls into the category of low-melting alloys. It boasts an optimal density of 6390 kg/m3, significantly enhancing its performance attributes, and making it highly recommended for steel processing in ladle applications.
References
1. Leont’ev L.I., Zayakin O.V., Volkov A.I. Problems of the metallurgical industry development to ensure the Russian technological sovereignty taking into account the mineral resource base state. Vestnik RAN. 2023;93(7):631–645. (In Russ.).
2. Leont’ev L.I., Zhuchkov V.I., Zayakin O.V., Sychev A.V., Mikhailova L.Yu. Potential for obtaining and applying complex niobium ferroalloys. Izvestiya. Ferrous Metallurgy. 2022;65(1):10–20. (In Russ.). https://doi.org/10.17073/0368-0797-2022-1-10-20
3. Posti A.I. Effect of micro alloying of steel with niobium on the mechanical properties of heat-strengthened rebar. Lit’e i metallurgiya. 2021;(1):73–77. (In Russ.). https://doi.org/10.21122/1683-6065-2021-1-73-77
4. Sun L.-y., Liu X., Xu X., Lei S.-w., Li H.-g., Zhai Q.-j. Review on niobium application in microalloyed steel. Journal of Iron and Steel Research International. 2022;29: 1513–1525. https://doi.org/10.1007/s42243-022-00789-1
5. Yan P., Bhadeshia H. Austenite–ferrite transformation in enhanced niobium, low carbon steel. Materials Science and Technology. 2015;31(9):1066–1076. https://doi.org/10.1179/1743284714Y.0000000673
6. Nayak S.S., Misra R.D., Hartmann J.E. Microstructure and properties of low manganese and niobium containing HIC pipeline steel. Materials Science and Engineering: A. 2008;494(1–2):456–463. https://doi.org/10.1016/j.msea.2008.04.038
7. Akhtar M.N., Khan M., Khan S.A., etc. Determination of non-recrystallization temperature for niobium microalloyed steel. Materials. 2021;14(10):2639. https://doi.org/10.3390/ma14102639
8. Guo A., Misra R.D.K., Xuet J., Guo B., Jansto S.G. Ultrahigh strength and low yield ratio of niobium-microalloyed 900 MPa pipeline steel with nano/ultrafine bainitic lath. Materials Science and Engineering: A. 2010; 527(16-17): 3886–3892. https://doi.org/10.1016/j.msea.2010.02.067
9. Matrosov M.Yu., Efron L.I., Kichkina A.A., Lyasotskii I.V. A study of the microstructure of niobium-microalloyed pipe steel after different modes of controlled rolling with accelerated cooling. Metal Science and Heat Treatment. 2008;50(3-4):
10. –141. https://doi.org/10.1007/s11041-008-9034-3
11. De Ardo A.J. Niobium in modern steels. International Materials Reviews. 2003;48(6):371–402. https://doi.org/10.1179/095066003225008833
12. Zhuchkov V.I., Noskov A.S., Zav’yalov A.L. Dissolution of Ferroalloys in Liquid Metal. Sverdlovsk: Ural Branch of the USSR Academy of Sciences; 1990:134. (In Russ.).
13. Zhuchkov V.I., Zayakin O.V. Development of composition and process of obtaining multicomponent ferroalloys. Izvestiya. Ferrous Metallurgy. 2020;63(10):791–795. (In Russ.). https://doi.org/10.17073/0368-0797-2020-10-791-795
14. Emlin B.I., Gasik M.I. Handbook of Electrothermal Processes. Moscow: Metallurgiya; 1978:288. (In Russ.).
15. Gasik L.N., Ignat’ev V.S., Gasik M.I. Structure and Quality of Industrial Ferroalloys and Alloys. Kiev: Tekhnika; 1975:128. (In Russ.).
16. Stroganov A.I. Requirements for ferroalloys for deoxidation and alloying. In: Transactions of Siberian Metallurgical Institute: Production of Ferroalloys. Novokuznetsk: Izd. Kuz. PI; 1980:5–24. (In Russ.).
17. Ignat’ev V.S., Bespalova I.A., Tikhorevsky V.S., etc. Physical properties of alloying alloys. In: Ferroalloy Production, ser. 5, Chermetinformatsiya. Vol. 2. Moscow; 1973:16. (In Russ.).
18. Zhuchkov V.I., Vatolin N.A., Zav’yalov A.L. On melting temperatures of ferroalloys. Metally. 1982;(4):45–46. (In Russ.).
19. Parimonchik I.B., Kazachkov I.P., Rezchik V.G. Modeling of ferroalloys dissolution in a steel ladle. Metallurgiya i koksokhimiya. 1972;(31):62–65. (In Russ.).
20. Petrov A.F., Kuksa O.V., Golovko L.A., Khodotova N.E. Prediction of physicochemical and thermophysical properties of standard grade ferroniobium. In: Fundamental and Applied Problems of Ferrous Metallurgy: Coll. of Sci. Papers. Dnіpro: ICM of the National Academy of Sciences of Ukraine; 2017;31:260–265. (In Russ.).
21. Kel I.N., Zhuchkov V.I., Renev D.S., Lozovay E.Y., Galiahmetova R.I. Study of the physicochemical characteristics of complex boron-containing ferroalloys. AIP Conference Proceedings. 2020;2313(1):050015. https://doi.org/10.1063/5.0032689
22. Li K.W., Wang X.B., Li S.M., Wang W.X., Chen S.P., Gong D.Q., Cui J.L. Halo formation in binary Fe-Nb off-eutectic alloys. High Temperature Materials and Processes. 2015; 34(5):479–485. https://doi.org/10.1515/htmp-2014-0081
23. Chemical Portal. Available at URL: https://himikatus.ru/art/phase-diagr1/Fe-Nb.php (Accessed: 18.10.2023). (In Russ.).
About the Authors
O. V. ZayakinRussian Federation
Oleg V. Zayakin, Corresponding Member of RAS, Dr. Sci. (Eng.), Chief Researcher, Head of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
I. N. Kel’
Russian Federation
Il’ya N. Kel’, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
D. S. Renev
Russian Federation
Dmitrii S. Renev, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. V. Sychev
Russian Federation
Aleksandr V. Sychev, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. Yu. Mikhailova
Russian Federation
Lyudmila Yu. Mikhailova, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. V. Dolmatov
Russian Federation
Aleksei V. Dolmatov, Cand. Sci. (Chem.), Scientific Secretary, Senior Researcher of the Laboratory of Metallurgical Melts
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Zayakin O.V., Kel’ I.N., Renev D.S., Sychev A.V., Mikhailova L.Yu., Dolmatov A.V. Physicochemical characteristics of new complex niobium-containing alloys. Izvestiya. Ferrous Metallurgy. 2023;66(5):616-622. https://doi.org/10.17073/0368-0797-2023-5-616-622