Scroll to:
Effect of boric anhydride on viscosity of slags used in electric melting of metallized siderite concentrate
https://doi.org/10.17073/0368-0797-2023-5-597-603
Abstract
The Bakal deposit located in the Southern Urals near the city of Bakal, Chelyabinsk region, is one of the largest deposits of carbonate iron ores (siderites). The total deposit of siderites is about 1 billion tons. They are not in demand among metallurgists because of their low iron content and high magnesium content. At the same time, the Urals metallurgical enterprises are suffering from shortage of iron ore raw materials including steelmaking ore raw materials. The high purity of siderites in terms of phosphorus and non-ferrous metals makes it possible to use methods of coke-free metallurgy for their processing. Pyrometallurgical processing of siderites including their reduction roasting in a rotary furnace followed by grinding and magnetic separation allows obtaining a concentrate to be used as a steelmaking raw material having metallization degree above 90 % and a waste rock content under 3 – 7 %. Calculations showed that the costs of electricity used for melting scrap metal and metallized siderite concentrate containing 30 % of waste rock and loaded into the furnace at temperatures above 1000 °C are close. We propose a siderite processing method including reduction of the initial ore in a rotary furnace, and melting of resulting metallized concentrate hot loaded (at temperatures above 1000 °C) into a furnace. The empty rock of metallized siderite concentrate contains a large percentage of magnesium oxide that makes it refractory. To obtain liquid slag, it is proposed to add boric anhydride in the form of colemanite. To assess the B2O3 effect on melting of the metallized siderite oxide phase in the process of electric melting, studies on the viscosity correlation of the magnesian steelmaking slag containing B2O3 with temperature and its composition were carried out. It was found that at the discharge temperature (1600 °C) the resulting magnesia slag with the ratio of MgO/SiO2 in the initial siderite equaling to 0.75 – 1.25 has a low viscosity (less than 3.65 P).
Keywords
For citations:
Vusikhis A.S., Leont’ev L.I., Mikheenkov M.A. Effect of boric anhydride on viscosity of slags used in electric melting of metallized siderite concentrate. Izvestiya. Ferrous Metallurgy. 2023;66(5):597-603. https://doi.org/10.17073/0368-0797-2023-5-597-603
Introduction
Carbonate (siderite) iron ore deposits span across various regions worldwide, including Austria, Bulgaria, Great Britain, Germany, Poland (Europe); China, Russia, Japan (Asia); Algeria (Africa); USA, Canada, Colombia (America), and numerous other countries [1 – 10]. Presently, the sole metallurgical process employing siderite ores is blast furnace smelting. Before loading into the furnace, siderites undergo enrichment, utilizing various methods depending on the ore’s composition: gravity, flotation, magnetic separation, electrostatic separation, and roasting-magnetic techniques. One of the largest known deposits of siderite ore globally is the Bakal deposit, situated near the city of Bakal in the Chelyabinsk Oblast, located in the Southern Urals. The total reserves of siderites in this deposit are estimated to be around 1 billion tons [11; 12]. However, due to their low quality – characterized by low iron content and high magnesium oxide content – siderites are in minimal demand among blast furnace metallurgists. Extraction of the ore falls significantly short of the potential yield based on mining and geological conditions. This shortage exacerbates the scarcity of raw materials faced by the metallurgical industry in the Urals, particularly for steelmaking.
Bakal siderites stand out due to their manganese content, reaching up to 2 %, low phosphorus content (less than 0.02 %), and the absence of non-ferrous metals like copper and zinc. These characteristics render them valuable raw materials for the production of high-quality steels using coke-free metallurgy methods [13; 14].
The methods utilized for direct iron production rely heavily on the quality of the iron ore raw materials employed. The recovery process of high-grade concentrates containing a minimum of 70 % iron is executed in various units such as shaft furnaces, retorts, among others. This procedure aims to achieve a metallization level surpassing 90 % using a specialized gas mixture [15]. For the treatment of low-grade ores, prevalent methods involve their metallization through solid reducing agents in rotary kilns, subsequently followed by the separation of waste rock via grinding and magnetic separation [16]. Extensive research indicates that through the pyrometallurgical enrichment of siderite ores [11; 12], a concentrate boasting over 90 % metallization can be attained. This concentrate, with a waste rock content ranging from 3 to 7 %, proves suitable for steelmaking purposes [17]. In order to facilitate the reduction process at temperatures between 1300 and 1350 °C, the easily fusible waste rock, predominantly composed of quartz-clay shale [19], necessitates prior removal. This removal can be achieved through gravity concentration in heavy suspensions [10], polygradient magnetic separation, or the X-ray radiometric method.
The comparison of energy intensity between smelting scrap metal in an electric furnace and smelting metalized siderite concentrate, heated to 1000 °C, containing approximately 30 % waste rock, revealed similar energy consumption per ton of iron in both scenarios. This similarity suggests the feasibility of a smelting technology for metalized siderite concentrate, acquired through pyrometallurgical enrichment in a rotary furnace, without the intermediary stages of grinding and magnetic separation. However, it’s crucial to consider that the high magnesium oxide content in the oxide phase of the concentrate will result in the formation of slag during the melting process with a considerably high melting point. This particular characteristic of the slag formation renders the proposed technology inefficient.
The addition of boric anhydride (B2O3 ) to slag is recognized for its capacity to lower the melting temperature of the slag [20]. To assess the impact of B2O3 on the melting behavior of the oxide phase of metalized siderite during the electric melting process, research focused on examining the correlation between the viscosity of magnesia steelmaking slag – incorporating B2O3 – and its temperature alongside composition.
Materials and methods
A vibrating viscometer, utilizing damped oscillations and computerized data processing, was employed for the viscosity study [21].
The proportion of various oxides (iron, calcium, aluminum, manganese) in siderites shows slight variation depending on their location. However, the ratio between silicon oxide and magnesium oxide can fluctuate from 0.5 to 1.25 [11; 12]. Consequently, to examine the viscosity of pure oxides, initial mixtures were formulated. These mixtures replicated the composition of the oxide phase found in a metallized siderite concentrate with a metallization degree of 95 %. The compositions maintained a constant proportion of most oxides while adjusting the SiO2/MgO ratio within the range of 0.5 to 1.25 (Table 1). These mixtures were supplemented with a material – previously molten and crushed – with a composition closely resembling calcined colemanite. This material contained 8 % SiO2, 34 % CaO, 4 % MgO and 54 % B2O3 . It was added at ratios equivalent to 10, 15, and 20 % of its fraction in the charge. This corresponds to 60, 90 and 120 kg of raw colemanite (L.O.I. 30 %) per ton of metallized siderite concentrate. The chemical composition of the examined slags is detailed in Table 2.
Table 1. Chemical composition of the initial mixtures
Table 2. Chemical composition of the studied slags
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The research involved forming tablets from mixtures mirroring the slag compositions under investigation. These tablets were placed in molybdenum crucibles, heated in a resistance electric furnace up to 1600 °C, and subsequently, the viscosity was measured.
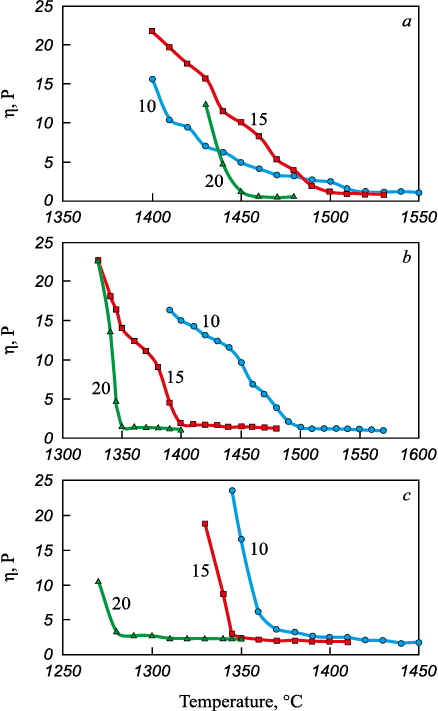
The analysis revealed that slags 1 – 3, featuring a SiO2/MgO ratio of 0.5, exhibited heterogeneity at temperatures below 1600 °C. In the other slags, distinct regions of high and low-temperature viscosity were observed. Within the high-temperature region, viscosity remained below 3.65 P. As the temperature decreased, viscosity showed a slight increase. However, in the low-temperature region, the change in viscosity was more pronounced. An elevation in the SiO2/MgO ratio and the addition of colemanite to the mixture led to an increase in B2O3 content in the slag and consequently lowered the temperature at which viscosity transitioned to the high-temperature region. The results of these measurements are depicted in Fig. 1.
Fig. 1. Change in viscosity of steelmaking slag depending |
To analyze the slag viscosities corresponding to the transition from the low-temperature to high-temperature regions, and the respective transition temperatures, experimental design methods were employed [22]. An orthogonal design 23 was used, with the SiO2/MgO ratio as the first factor and the colemanite fraction in the charge as the second factor. The experimental plan and its outcomes are detailed in Table 3 and presented graphically in Figs. 2 – 4.
Table 3. Plan of the experiment and its results
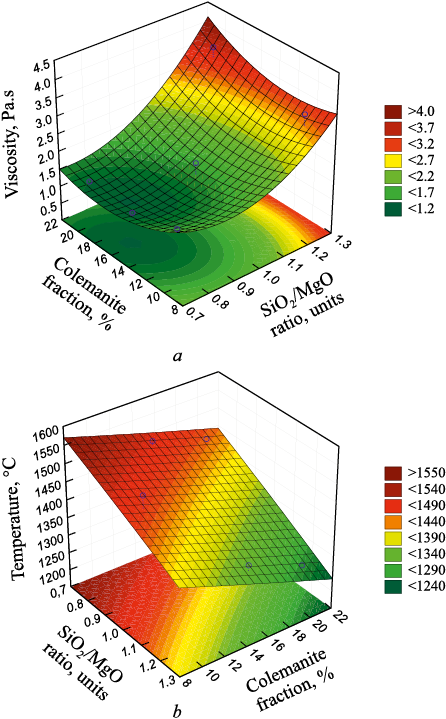
Fig. 2. General view of slag viscosity response function (a) |
Fig. 2 provides an overview of the response function depicting slag viscosity and the temperature at which the desired viscosity is achieved, in relation to the SiO2/MgO ratio and colemanite content.
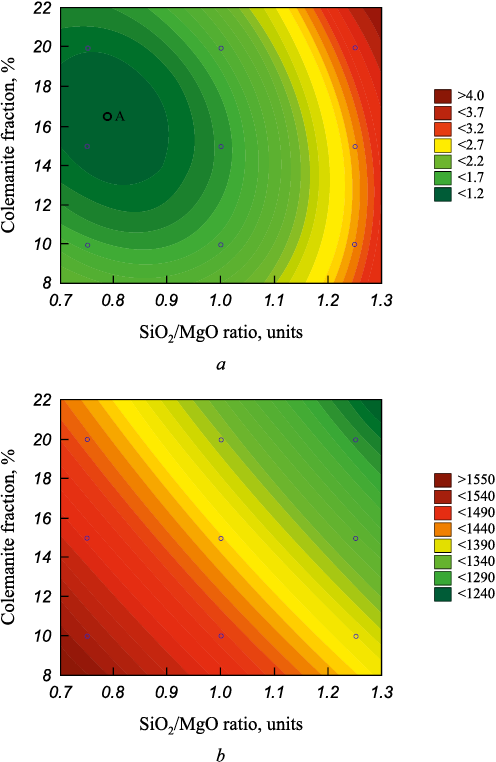
Fig. 3. Isolines of equal slag viscosity (a) and equal temperature |
Fig. 3 shows isolines representing equivalent slag viscosities and the respective temperatures required to achieve those viscosities, contingent on the SiO2/MgO ratio and colemanite content.
The analysis depicted in Figs. 2 and 3 elucidates the profound impact of the SiO2/MgO ratio and colemanite content on viscosity. Point A in Fig. 3 marks the minimum slag viscosity, attained with a SiO2/MgO ratio of 0.78 and a colemanite content of 17 %. At a consistent SiO2/MgO ratio, an increase in colemanite content results in a decrease in the temperature needed to achieve the desired viscosity. For instance, the temperature required to achieve the minimum viscosity with a SiO2/MgO ratio of 0.78 and a 17 % colemanite content is 1460 °С.
Using the STATISTICA software [24], the experimental results were processed, enabling the calculation of regression equations describing the behavior of the response function (viscosity (1) and temperature (2)) based on the main factors:
| η = 10.93 – 15.58x + 8.3x2 – 0.4y + 0.009y2 + 0.15xy; | (1) |
| Т = 1801.7 – 199.5x – 28.6x2 – 7.19y + 0.03y2 – 4.0xy, | (2) |
where x is the SiO2/MgO ratio, units; y is the colemanite content, wt. %.
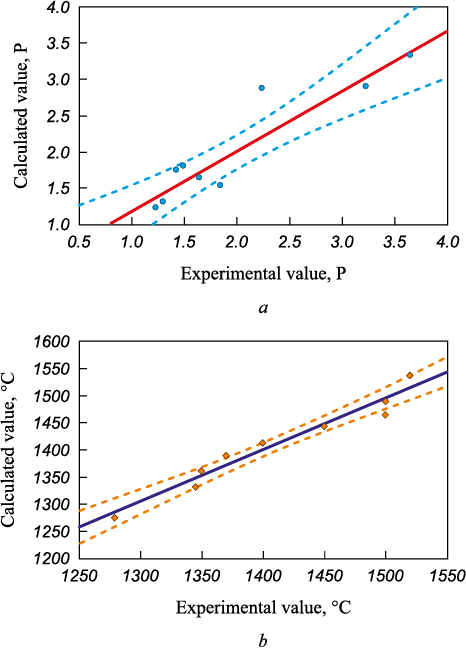
Fig. 4 illustrates the comparison between experimental and calculated values of viscosity and temperature to evaluate the model’s adequacy determined by the regression equation. The results, assessed via correlation of experimental and calculated data, are visually represented in this figure. The data analysis demonstrates that the regression equation effectively describes the experimental findings. Most of the experimental data points fall within the confidence interval demarcated by the reliability ellipse.
Fig. 4. Correlation diagram of experimental and calculated values |
The findings confirm that when the SiO2/MgO ratio stands at 0.5, the inclusion of colemanite does not yield favorable outcomes. Below temperatures of 1600 °C, the slag retains heterogeneity, impeding the smelting of metallized siderite in electric furnaces. Conversely, in slags featuring a SiO2/MgO ratio surpassing 0.75, a high-temperature region with viscosity measuring less than 3.65 P is evident. This corresponds to temperatures exceeding 1520 °C, with colemanite fractions ranging from 10 to 20 %. The transition temperature from high to low-temperature regions can be managed by altering both the SiO2/MgO ratio and the colemanite fraction. Specifically, achieving the same transition temperature involves concurrently increasing the SiO2/MgO ratio while decreasing the colemanite fraction.
Conclusions
Lump siderite concentrate, reduced to a metallization degree of 95 % in a rotary kiln and loaded into an electric furnace at hot temperatures (above 1000 °C), supplemented with raw colemanite additives within the range of 60 – 120 kg per ton of concentrate, can undergo melting. This process yields a metal-semi-product at outlet temperatures around 1600 °C, suitable for subsequent steel production. Simultaneously, it results in the formation of a homogeneous magnesia slag exhibiting low viscosity (below 3.65 P). This occurs specifically when the original siderite boasts an MgO/SiO2 ratio ranging from 0.75 to 1.25.
References
1. Frimmel H. Strontium isotopic evidence for the origin of siderite, ankerite and magnesite mineralizations in the Eastern Alps. Mineralium Deposita. 1988;23(4):268–275. https://doi.org/10.1007/BF00206407
2. Pohl W. Comparative geology of magnesite deposits and occurrences. In: Magnesite. Geology, Mineralogy, Geochemistry, Formation of Mg-Carbonates. Moeller P. ed. Monogr. Ser. Mineral Deposits. 1989;28:1–13.
3. Jonkov J., Mochev D., Grigorova I., Nishkov I. Siderite ore physical separation and reverse flotation. Journal of Mining and Metallurgy A: Mining. 2012;48:23–37.
4. Boch R., Wang X., Kluge T., etc. Aragonite–calcite veins of the ‘Erzberg’ iron ore deposit (Austria): Environmental implications from young fractures. Sedimentology. 2019;66(4):604–635. https://doi.org/10.1111/sed.12500
5. Min L., Zhiming C., Qiying C. The role of organic matter in the formation of siderite from Xuanlong area, Hebei Province. Chinese Journal of Geochemistry. 1997;16: 86–94. https://doi.org/10.1007/BF02843376
6. Bai S., Wen S., Liu D., Zhang W., Xian Y. Catalyzing carbothermic reduction of siderite ore with high content of phosphorus by adding sodium. ISIJ International. 2011;51(10): 1601–1607. https://doi.org/10.2355/isijinternational.51.1601
7. Shikazono N. Composition of siderite and the environments of formation of vein-type deposits in Japan. Economic Geology.1977;72(4):632–641. https://doi.org/10.2113/gsecongeo.72.4.632
8. Palinkas S.S., Spangenberg J.E., Palinkas L.A. Organic and inorganic geochemistry of Ljubija siderite deposits, NW Bosnia and Herzegovina. Mineralium Deposita. 2009;44: 893–913. https://doi.org/10.1007/s00126-009-0249-z
9. Rudmin M.A., Kalinina N.A., Maximov P.N. Formation of siderite in marine ooidal ironstones on example of Bakchar Deposit (Western Siberia). In: Proceedings of the X Int. Siberian Early Career Geoscientists Conf. Novosibirsk State University; 2022:79–80.
10. Zhunev A.G., Yur’ev B.P., Blank M.E. Intensification of roasting and agglomeration of siderite ores. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 1988;(3):2–13. (In Russ.).
11. Yur’ev B.P., Melamud S.G., Spirin N.A., Shatsillo V.V. Technological and Thermal Engineering Bases of Siderite Ore Preparation for Metallurgical Processing: Monograph. Yekaterinburg: Den’ RA; 2016:428. (In Russ.).
12. Vusikhis A. S., Leont’ev L. I. The Use of Siderite Ores in Production of Iron and Steel: Monograph. Moscow, Vologda: Infra-Inzheneriya; 2022:116. (In Russ.).
13. Lapteva A. Coke-free metallurgy: Resources, state, prospects. Metally Evrazii. 2012;(2):9–23. (In Russ.).
14. Kirichenko I.S., Aleksakhin A.V. Development of global and domestic production of direct reduced iron. Molodoi uchenyi. 2016;(2):85-90. (In Russ.).
15. Timoshpol’skii V.I., Trusova I.A., Plyushchevskii I.N., Korneev S.V. Prospects for the production and use of metallized raw materials to obtain high-quality steel grades. Message 1. Analysis of modern schemes for obtaining metallized raw materials. Lit’e i metallurgiya. 2009;(50):134–138. (In Russ.).
16. Kurunov I.F., Savchuk N.A. State and Prospects of Direct Metallurgy of Iron. Moscow: Chermetinformatsiya; 2002:186. (In Russ.).
17. Gimmel’farb A.I., Nemenov A.M., Tarasov B.E. Metallization and Electric Smelting of Iron Ore Raw Materials. Moscow: Metallurgiya; 1981:152. (In Russ.)
18. Yur’ev B.P., Sheshukov O.Yu., Dudko V.A. Elaboration of an environmentally appropriate technology of siderite ores concentration. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2019;75(8):923–929. (In Russ.). https://doi.org/10.32339/0135-5910-2019-8-923-929
19. Akhlyustina A.I., Zhukovskii G.V., Kvaskov A.P. Technological classification of iron ores of the Bakal deposit. Trudy “Uralmekhanobr”. 1972;(18):37–43. (In Russ.).
20. Ren Sh., Zhang J., Wu L., Fu W., Rao J. Influence of B2O3 on viscosity of high Ti-bearing blast furnace slag. ISIJ International. 2012;52(6):984–991. https://doi.org/10.1051/metal/2016038
21. Vusikhis A.S., Selivanov E.N., Dmitriev A.N., Chentsov V.P., Ryabov V.V. Structure sensitive properties of system B2O3–CaO melts. Defect and Diffusion Forum. 2020;400:186–192. https://doi.org/10.4028/www.scientific.net/DDF.400.186
22. Kuprienko N.V., Ponomareva O.A., Tikhonov D.V. Statistics. Distribution Analysis Methods. Selective Observation: Tutorial. St. Petersburg: Izd-vo Politekhn. un-ta; 2009:138. (In Russ.)
About the Authors
A. S. VusikhisRussian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. I. Leont’ev
Russian Federation
Leopol’d I. Leont’ev, Academician, Adviser, Russian Academy of Sciences, Dr. Sci. (Eng.), Prof., National University of Science and Technology “MISIS”, Chief Researcher, Institute of Metallurgy, Ural Branch of the Russian Academy of Science
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
32a Leninskii Ave., Moscow 119991, Russian Federation
M. A. Mikheenkov
Russian Federation
Mikhail A. Mikheenkov, Dr. Sci. (Eng.), Senior Researcher of the Laboratory “Pyrometallurgy of Ferrous Metals”
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Vusikhis A.S., Leont’ev L.I., Mikheenkov M.A. Effect of boric anhydride on viscosity of slags used in electric melting of metallized siderite concentrate. Izvestiya. Ferrous Metallurgy. 2023;66(5):597-603. https://doi.org/10.17073/0368-0797-2023-5-597-603