Scroll to:
Electrochemistry of reduction processes and prospects for the development of reduction technologies
https://doi.org/10.17073/0368-0797-2025-4-424-433
Abstract
Oxidation and reduction of metals consist in the loss of valence electrons by metal atoms with the conversion of an electromagnetic metallic bond into an ionic bond during oxidation and the reverse transition of electrons from anions to metal cations with the conversion of an ionic bond into a metallic bond during reduction. The electronic reduction theory developed by the authors describes the reduction process by the sequential operation of two electrochemical cells: a fuel cell, in which the chemical energy of the oxidized reducing agent is converted into electrical energy of “free” electrons, and a solid electrolyte electrolyzer, which converts the electrical energy of these electrons into energy of the metal bond of the reduced cations in the oxide. Since the stage of the actual reduction is the formation of a metallic bond between cations due to electrons coming from outside, the shortest and therefore most effective supply of electrons to the reduced cations will be not from the fuel cell, but from the electrical network, that is, electrolysis of the oxides of the metal being reduced. Known methods for producing iron by electrolysis of molten oxides, as well as possibly alkaline solutions, are promising for extracting iron from rich ores. For the selective extraction of iron from ferromanganese, titanomagnetite, siderite, chromite and other complex ores, relatively low-temperature reduction of iron with hydrogen or solid-phase electrolysis to produce, after separation, a metallization product by melting carbon-free iron and a concentrate of active metal oxides is more promising.
Keywords
For citations:
Roshchin V.E., Roshchin A.V. Electrochemistry of reduction processes and prospects for the development of reduction technologies. Izvestiya. Ferrous Metallurgy. 2025;68(4):424-433. https://doi.org/10.17073/0368-0797-2025-4-424-433
Introduction
Contemporary methods for reducing and extracting metals from ores long predate the rise of science and took shape through the gradual refinement of the craft of metal production. In fact, the practice of turning ore into metal largely motivated the emergence of a science of material transformation – first as alchemy and later as chemistry. Only at the end of the 19th century, with the development of atomic–molecular concepts grounded in advances in the science of chemical reactions, the first scientific explanations of the mechanisms operating in metal reduction appear [1 – 3].
There is now little doubt that atoms and molecules exist only in the gas phase: condensed metals contain no discrete atoms, and ore oxides contain no molecules. In both media, metals are present as cations and the valence electrons “lost” by the atoms; these electrons, by means of the electromagnetic field, bind the metal cations into the condensed phase – metallic bonding in metals and ionic bonding in oxides. It is likewise clear that oxidation and reduction of metals amount to a redistribution of atomic valence electrons. These processes should therefore be viewed as reactions in which metal atoms lose valence electrons to oxidizer atoms, with metallic electromagnetic bonding giving way to ionic bonding during oxidation (Ме 0 = Ме 2+ + 2е), and as the return of these electrons from oxidizer anions to the cations during reduction, restoring metallic bonding (Ме 2+ + 2е = Ме 0 ). Thus, reduction and oxidation are electrochemical processes driven by the redistribution of the valence electrons of metal atoms. Nevertheless, in metallurgical science the description of ore-to-metal reduction processes is still dominated by the atomic–molecular approach [4; 5]. An obviously outdated atomistic description of reduction at the atomic–molecular level – for example, С + FeO = CO + Fe – does not, by itself, support adequate improvement of industrial technologies or their alignment with the current state of science [6 – 8].
Electronic theory of reduction
Developed in recent years by the authors and their colleagues, the electronic theory of reduction [9; 10] aims to describe the electrochemistry of electron exchange between the oxidized atoms of the reductant and the cations of the metal being reduced. The theory rests on a large number of dedicated experiments conducted by the authors and on a synthesis of published results by others. It proceeds from the electrochemical nature of reduction reactions, accounts for structural changes in solid ores (formation of anion vacancies under low oxygen partial pressure PO2 and high temperature Т ≈ 2000 °С), and for composition of the gas phase corresponding to these conditions (formation of a low-temperature plasma) during heating in reduction units. It is grounded in the physics of imperfect crystals and in quantum-mechanical principles governing the distribution and motion of electrons in metals and ionic semiconductors. The theory’s conclusions encompass all known reduction outcomes: metal formation on the surfaces of rich monomineralic ore lumps; metal precipitation within complex and lean ores; oxide dissociation; formation and sublimation of suboxides; and metal production by electrolysis from aqueous solutions and molten salts.
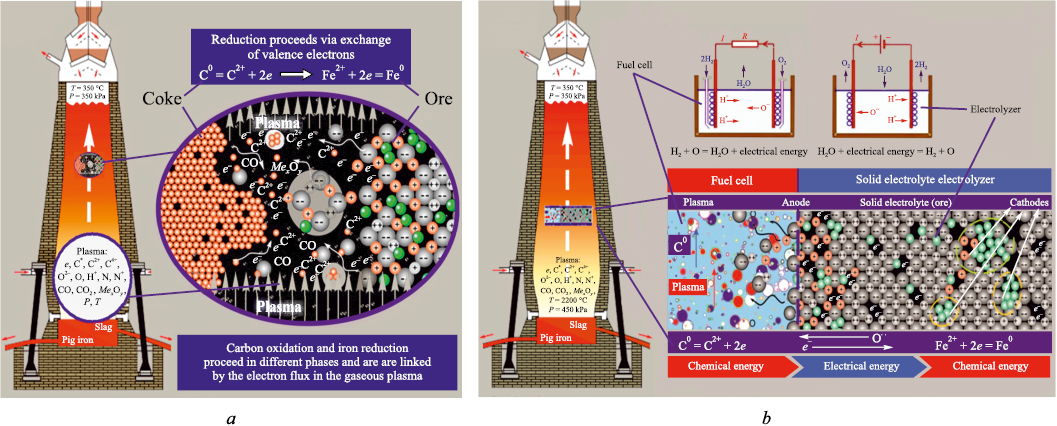
According to the theory, at high temperature and low oxygen partial pressure in the gas phase of reduction units, chemical reactions between solid ore oxides and the solid carbon of coke do not even require direct contact between the solid reagents. The plasma state of the gas phase enables ions and electrons present both in the plasma and on the surfaces of the solids in contact with it to interact over a distance under electrostatic forces, approach one another, and react in the gas phase. This, in turn, allows solid-state reduction reactions to proceed rapidly in the kinetic mode (Fig. 1, a).
Fig. 1. Electrochemical processes in a blast furnace: |
A new and fundamentally important element of the electronic theory of reduction – derived theoretically and confirmed repeatedly by dedicated experiments with complex and lean ores – is the mechanism of electron transfer from the reductant to the cations of the metal being reduced, i.e., the mechanism of solid-state reduction. It involves the formation and migration, within the crystal lattice of each oxide lump, of anion vacancies bearing “free” electrons (not bound to any specific cation). These electrons originate in the chemical reaction between the reductant and the oxygen of the oxide in the gas phase. The low oxygen partial pressure in the gas phase, established by chemical interaction with the reductant, together with the high mobility of lattice ions at elevated temperature, causes oxygen to leave the oxide and enter the gas phase. As an oxygen atom departs the oxide lattice and accepts two electrons from the reductant in the gas phase, it leaves behind an anion vacancy accompanied by two “free” electrons previously taken by the oxygen anion from the metal cations.
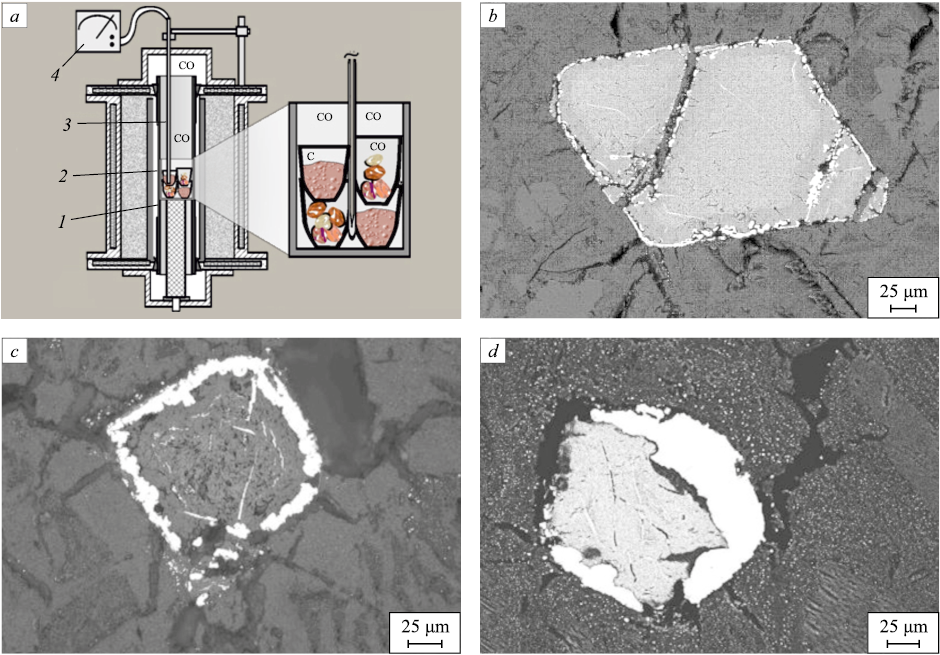
Owing to vigorous thermal motion of ions, these anion vacancies and their associated “free” electrons disperse through the lattices of complex oxides and migrate until they encounter cations with high electron affinity (as determined by their Fermi-level position) [11]. Owing to vigorous thermal motion of ions, these anion vacancies and their associated “free” electrons disperse through the lattices of complex oxides and migrate until they encounter cations with high electron affinity (as determined by their Fermi-level position) [11]. Where such cations accumulate, the migration of the electron-charged vacancies ceases, the vacancies coalesce, and metallic bonding forms at those sites by virtue of the “free” electrons carried with the vacancies; a metallic phase then precipitates. Thus, removal of atomic oxygen from the surface of one oxide phase can lead to selective reduction of cations with high electron affinity within the crystal lattice of a different oxide. An example is shown in Fig. 2, which presents the scheme and results of experiments on solid-state reduction by solid carbon of iron and chromium cations in crystals of chromspinelide (chromian spinel) (Mg, Fe)[Fe, Al, Cr]2O4 embedded in olivine (Mg, Fe)2[SiO4 ].
Fig. 2. Scheme of the installation based on the Tammann furnace for conducting experiments |
The concerted migration of “free” electrons and anion vacancies under reducing conditions proceeds without an externally applied electric field because of gradients in the chemical potentials of oxygen and electrons between the surface and the bulk of the oxide phase. “Free” electrons may also move via anion vacancies of other origin (thermal or impurity-related), provided that overall and local charge neutrality is preserved.
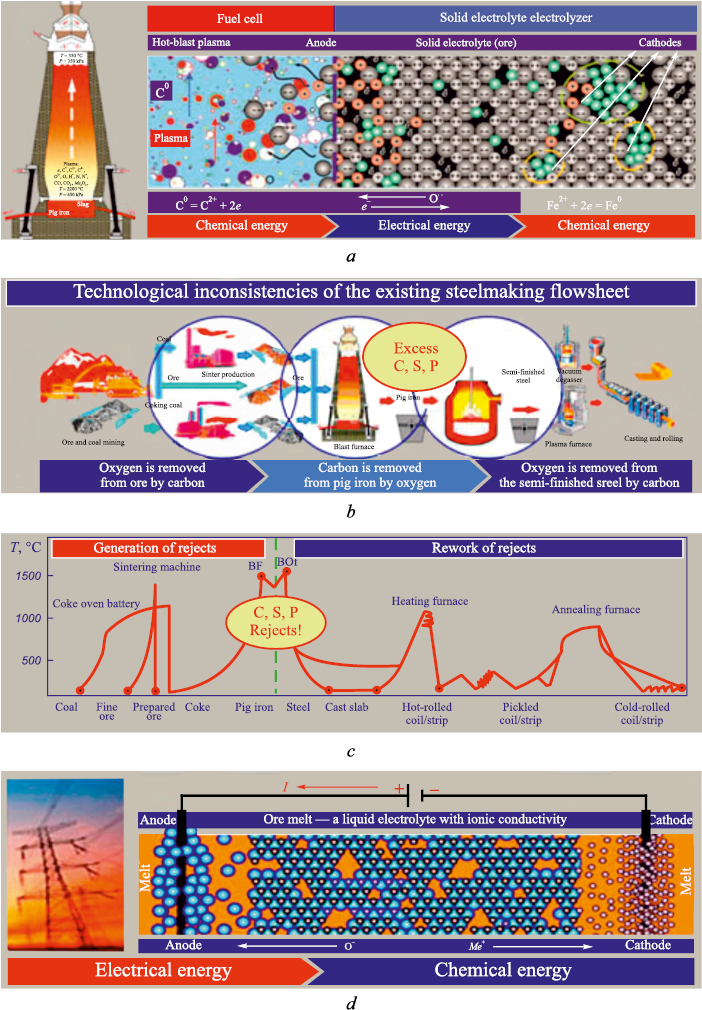
Accordingly, the reduction process in industrial reduction units should be viewed as the combined action of two electrochemical cells operating in series: a carbon fuel cell, in which the chemical energy of the oxidized reductant is converted into the electrical energy of “free” electrons; and a solid electrolyte electrolyzer, in which the electrical energy carried by electrons that have migrated from the surface into the bulk of the oxide is taken up by cations and converted into the chemical energy of metallic bonding (Fig. 1, b). In reduction units, the gas phase and each individual ore lump together form such series-connected pairs of electrochemical cells (fuel cells and electrolyzers). Because the number of these cell pairs – one per ore lump – is enormous, reduction units – and blast furnaces in particular – achieve exceptionally high throughput.
Prospects for transforming carbon-based reduction technologies
The electrochemical nature of oxidation/reduction processes should no longer be a matter of debate. It follows that there must be a flow of electrons in reduction units between the reductant and the cations of the metal being reduced – that is, an electric current between them. The electronic theory describes how this current arises and is transformed as the result of two electrochemical cells operating in series: an electrochemical fuel cell that converts chemical energy into electrical energy, and an electrolyzer. This mechanism is consistent with the operating practice of all types of industrial reduction units and is supported by numerous dedicated experiments using different ores and different reductants. From this, one may conclude that the final stage of today’s multistage metal-reduction technologies is invariably the transfer of “free” electrons from atoms of the reductant to the cations of the metal being reduced, with atomic oxygen being released at the interfaces – on the surfaces of the oxide and of the metal within the oxide phase – i.e., solid-electrolyte electrolysis of the ore.
This raises the question of whether the many costly preliminary and intermediate stages of the reduction process are necessary, and whether it is sensible to generate “free” electrons in the fuel-cell portion of ore-reduction units at all. It is evidently far simpler and less expensive to generate electrons not by burning the reductant in metallurgical reduction units, but by modern methods at power plants, transmit them through the electrical grid, and use them directly at the final stage – the reduction of cations – in a specialized electrochemical unit (an electrolyzer). Accordingly, the existing multistage, energy-intensive process of extracting iron from ores (Fig. 3, a – c) should be collapsed to a single concluding stage – electrolysis (Fig. 3, d). In this light, extracting metals from ores by electrolysis appears to be the ultimate goal of transforming current metallurgical technologies.
Electrolysis of molten salts and oxides has long been used to reduce and produce several metals at industrial scale – for example, aluminum [12]. Work on developing units and processes for extracting iron by electrolysis from aqueous solutions of its salts has also been under way for quite some time [13]. In recent years, several companies have intensified research into units and processes for producing iron by electrolysis of molten oxides [14 – 16]. Overall, however, both practitioners – and, more importantly, the scientific community – remain not merely skeptical but largely negative about the prospects of obtaining iron by electrolysis.
Until now, the rationale for producing pig iron with subsequent conversion to steel has been the enormous throughput of blast furnaces and the availability and relatively low cost of carbonaceous materials used both as a heat source and as reductants. It is worth recalling that this technology arose and evolved historically from the need to rework “defective” product: as the temperature in the bloomery hearth increased and iron became saturated with carbon, brittle cast iron was obtained instead of low-carbon, ductile iron. Producing and then reworking this “defect” proved expedient because carbonaceous reductants were accessible and relatively inexpensive (first wood, then charcoal, and later coal). Today, however, the production of pig iron relies on the most expensive solid fuel – coke, obtained from scarce coal grades by a complex and environmentally hazardous process. As high-quality coking coals become scarce, coke prices keep rising, and meeting ever-stricter quality requirements is increasingly difficult. Moreover, because of how reduction proceeds in blast furnaces, no more than 25 % of the calorific value of this scarce and costly reductant can be used directly: during iron reduction the coke carbon is oxidized only to CO, and 75 % of the energy is released later during non-target “afterburning” of СО to СО2 [17].
This extremely low targeted (for reduction) utilization of coke is compounded by dissolution of a portion of carbon in iron, producing pig iron. The dissolved carbon is not merely a loss – it becomes a harmful impurity that must be removed when converting pig iron to steel by oxygen blowing, which simultaneously oxidizes an appreciable amount of iron that the coke had previously reduced in the blast furnace (Fig. 3, b). The oxidized melt then requires deoxidation, including with carbon. Taking into account the huge heat losses across the multistage route of turning a “defect” into the final product, the pig iron → steel process must be judged extremely inefficient, fundamentally inconsistent with energy and resource conservation, and out of step with the current state of science. During steelmaking, in addition to removing the root cause of the “defect” (excess carbon), it is also necessary to remove sulfur and phosphorus, introduced mainly at the pig-iron stage by coke and flux additions. In essence, the modern scheme of producing pig iron and then converting it to steel is a process of producing and correcting a defect (Fig. 3, b, c).
Fig. 3. Conversion and loss of energy when using a carbon fuel cell according |
Given the inconsistency of the existing production chain – alternating reduction/oxidation, contamination/refining, and heating/cooling (Fig. 3, c) – together with the costs of mining and transporting scarce coking coal and making coke, producing sinter and other consumables, building modern, gigantic reduction units, logistics, and environmental protection and compliance, the costs of electrolysis are likely not substantially different from those of producing and reworking pig iron. Producing iron by electrolysis of melts may already be competitive for greenfield plants (Fig. 3, d). As noted above, the enormous productivity of a blast furnace is achieved by the simultaneous operation within its volume of an immense number of solid electrolyte electrolyzers. A similar electroreduction principle – using grid electricity – may be applicable at the metallization stage.
Concerns about excessively high electricity costs for iron electrolysis also appear to be overstated. First, producing 1 kg of iron by electrolysis requires roughly four times less energy than producing the same mass of aluminum. Second, reserves of high-quality coking coals are rapidly declining, whereas the availability of comparatively inexpensive electric power is increasing with the expansion of nuclear energy. Tellingly, intensive research on the electrolytic production of iron is underway in the United States and France, countries with some of the highest shares of nuclear power generation [14 – 16].
Hydrogen reduction processes and technologies
The prospect of producing iron by electrolysis of ore melts appears entirely realistic for rich single-mineral ores. At the same time, there are vast reserves of complex and lean ores that are difficult or unsuitable for processing by existing methods. The advisability of preparing such ores for melt electrolysis also appears doubtful. Our studies [18] have shown that, in these ores, iron can be selectively reduced in the solid state, i.e., at relatively low temperature; after separating the products of metallization by melting, one can obtain carbon-free iron and a concentrate of oxides of metals with lower electron affinity than iron.
Accordingly, alongside producing iron by electrolysis from rich ores, it is also promising to investigate selective reduction of iron in lean and complex sideritic, iron–manganese, titanomagnetite, ilmenite, and other ores, followed – after separation of the metallization products – by obtaining iron and an oxide concentrate of reactive metals (magnesium, manganese, titanium, chromium, and others). The task is not only to maximize the reduction and recovery of iron, but also to maximize retention of valuable oxides of reactive metals in the oxide phase. Our results indicate that the most effective way to achieve this is to use hydrogen rather than carbon as the reducing agent [19].
On a large industrial scale, the only truly realistic alternative to carbon as a reducing agent for iron is hydrogen, the most abundant chemical element in the Universe. It is also among the most effective energy carriers: burning 1 kg of hydrogen releases about 140 MJ, whereas 1 kg of gasoline yields roughly 50 MJ, and 1 kg of coal 20 – 25 MJ. However, there is no free, concentrated hydrogen on Earth; to use hydrogen as an energy carrier or reducing agent, it must be produced by extracting it from abundant compounds – water or hydrocarbons. Depending on the carbon footprint of its production, hydrogen is labeled by color, from “green” (lowest footprint), obtained by electrolysis of water using renewable electricity, to “brown,” obtained by coal gasification. “Green” hydrogen has the smallest carbon footprint, but it is also the most expensive. Currently, the most accessible and least costly routes of hydrogen production are processes that split fossil hydrocarbons into hydrogen and carbon – resources with which Russia is well endowed.
Because of several properties – its gaseous state; its high affinity for oxygen at low temperature that decreases as temperature rises; its negligible solubility in solid iron, among others – hydrogen can in some cases be a more effective reducing agent, particularly for selective solid-phase reduction of iron in complex ores.
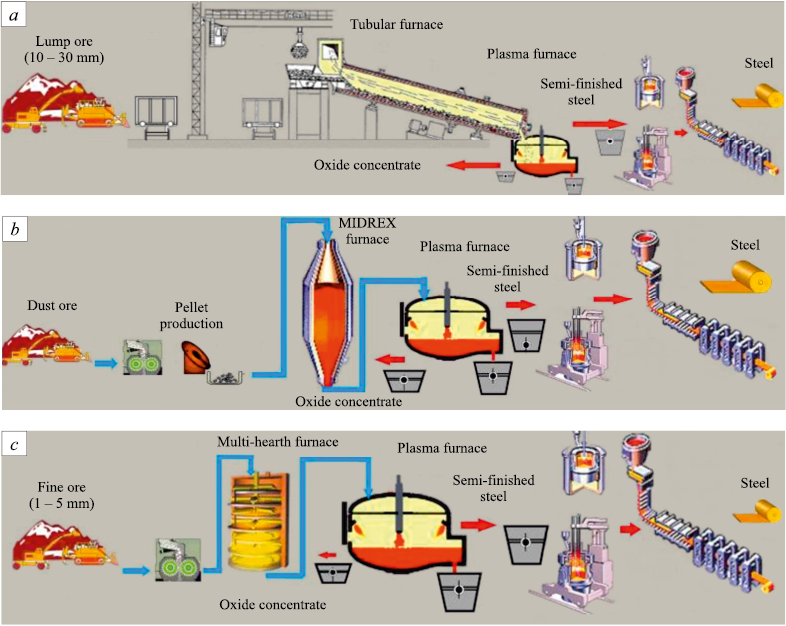
Gaseous hydrogen cannot replace solid fuel in a blast furnace, but it can readily be used as a reducing agent in existing direct-reduction units in place of natural gas (Fig. 4). As with carbon in the blast furnace, the chemical energy of hydrogen when oxidized by ore oxygen in the fuel-cell stage of the reduction unit, is converted into electrical energy, and that energy then drives metal reduction.
Fig. 4. Reasonable schemes for the use of hydrogen as a reducing agent for selective reduction |
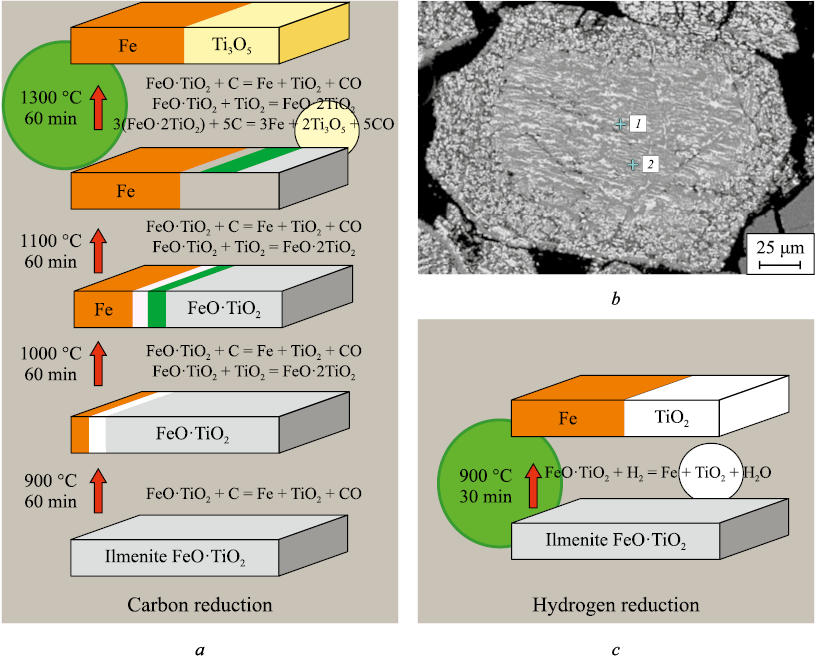
Analysis of the electrochemical processes based on a hydrogen solid electrolyte electrochemical fuel cell shows clear advantages of hydrogen over carbon for waste-free processing of complex ores that are difficult to treat by the blast-furnace route – ilmenite, titanomagnetite, iron–manganese, sideritic, and others – yielding new valuable products: carbon-free soft iron and valuable concentrates of oxides of titanium, manganese, magnesium, and other active metals [18; 19]. At present, for operating plants there is a commercially justified niche for using comparatively inexpensive “gray” or “brown” hydrogen to obtain carbon-free soft iron and valuable oxide concentrates from complex ores (Fig. 5).
Fig. 5. Comparison of sequence and parameters (temperature (T) and time (t)) |
As for the reduction of iron with “green” hydrogen, – produced by water electrolysis and often promoted as a panacea for dangerous climate change [20 – 23], – its role climate guarantor is doubtful, and its economic viability is unlikely. This is because using “green” hydrogen as a reducing agent entails sequential double electrolysis: first, electrolysis of the more stable oxide (water) using renewable electricity; second, electrolysis of the less stable iron oxide, driven by the electrical energy released in the hydrogen electrochemical fuel cell. Moreover, producing carbon-free iron via reduction with “green” hydrogen would still require subsequent carburization during melting in arc furnaces [24 – 26]. Accordingly, direct electrolysis of the less stable metal oxides remains the more rational route.
Conclusions
Metals do not contain discrete atoms, and ore oxides contain no molecules: in both media, metals are present as cations bound by the valence electrons of metal atoms – by metallic bonding in metals and by ionic bonding in ore oxides. Oxidation of metals is the loss of valence electrons by metal atoms; reduction is the return of those electrons to metal cations. In other words, oxidation and reduction are electrochemical processes.
The electronic theory of reduction describes the transfer of valence electrons from atoms of the reductant to the cations of the metal being reduced. Under the conditions of industrial reduction units, the gas phase is in a state of low-temperature plasma, while ore oxides exhibit mixed electronic–anion conductivity. As a result, electron exchange between reductants and metal cations in ores does not even require physical contact between the reductant and the ore.
The electronic theory of reduction describes the transfer of valence electrons from atoms of the reductant to the cations of the metal being reduced. Under the conditions of industrial reduction units, the gas phase is in a state of low-temperature plasma, while ore oxides exhibit mixed electronic–anion conductivity. As a result, electron exchange between reductants and metal cations in ores does not even require physical contact between the reductant and the ore.
In complex ores, the “free” electrons that form at the surface can migrate together with anion vacancies through the oxide crystal lattice until they encounter cations that, under the given conditions, have the highest electron affinity. There they become trapped; at coalesced anion-vacancy sites a metallic phase forms. In this way, oxygen departing to the gas phase from one oxide can lead to the reduction of cations within the crystal lattice of a different oxide.
By their physical operating principle, reduction units are electrochemical devices in which two familiar electrochemical cells act in series: a fuel cell, which converts the chemical energy of the reductant into the electrical energy of “free” electrons, and a solid electrolyte electrolyzer, which converts that electrical energy into the chemical energy of metallic bonding in the cations being reduced.
Since the final stage of any reduction reaction is the uptake of “free” electrons by the cations being reduced, the most direct route to reduction is to generate these electrons at modern power stations and deliver them to the cations via the electrical grid – that is, electrolysis. Electrolysis of melts for the production of carbon-free iron constitutes a promising pathway for processing rich, single-mineral iron ores. A comparably promising avenue for processing complex and lean ores is the selective, low-temperature reduction of iron using hydrogen, with subsequent separation to obtain carbon-free iron together with oxide concentrates of more active metals (e.g., manganese, chromium, titanium).
References
1. Lyuban A.P. Analysis of Blast Furnace Smelting Phenomena. Moscow: Metallurgizdat; 1962:532. (In Russ.).
2. Bogdandy L., Engell H.-J. The Reduction of Iron Ores. Scientific Basis and Technology. Berlin; Heidelberg: Springer-Verlag; 1971:576. https://doi.org/10.1007/978-3-662-10400-2
3. Wegman E.F., Zherebin B.N., Pokhvistnev A.N., etc. Metallurgy of Cast Iron. Moscow: Metallurgiya; 1989:512. (In Russ.).
4. Ghosh A., Chatterjee A. Ironmaking and Steelmaking: Theory and Practice. PHI Learning Pvt. Ltd.; 2008:472.
5. Dmitriev A.N., Shumakov N.S., Leont’ev L.I., Onorin O.P. Fundamentals of the Theory and Technology of Blast Furnace Smelting. Yekaterinburg: UB RAS; 2005:545. (In Russ.).
6. Korotich V.I., Naboichenko S.S., Sotnikov A.I., etc. Beginnings of Metallurgy: Textbook for Universities. Yekaterinburg: USTU; 2000:392. (In Russ.).
7. Konovalov Yu.V., Troyanskii A.A., Timoshenko S.N. Metallurgy: Textbook in three books. Book 1: Production of Cast Iron, Iron, Steel and Ferroalloys. Donetsk: DonSTU; 2011:431. (In Russ.).
8. Yusfin Yu.S., Pashkov N.F. Metallurgy of Iron. Moscow: Akademkniga; 2007:464. (In Russ.).
9. Roshchin V.E., Roshchin A.V. General electron theory of reduction and oxidation of metals. Izvestiya. Ferrous Metallurgy. 2020;63(3-4):271–285. https://doi.org/10.17073/0368-0797-2020-3-4-271-285
10. Roshchin V.E., Roshchin A.V. Physics of Pyrometallurgical Processes. Textbook. Moscow; Vologda: Infra-Engineering; 2021:304. (In Russ.).
11. Ponomarenko A.G. Issues of thermodynamics of phases of variable composition having a collective electronic system. IV. The Fermi level in oxide phases. Journal of Physical Chemistry. 1974;XLVIII(8):1954–1958. (In Russ.).
12. Galevskii G.V., Kulagin N.M., Mindis M.Ya., Sirazutdinov G.A. Metallurgy of Aluminum. Technology, Power Supply, Automation. Moscow: Flinta; Nauka; 2017:529. (In Russ.).
13. Lopes D.V., Quina M.J., Frade J.R., Kovalevsky A.V. Prospects and challenges of the electrochemical reduction of iron oxides in alkaline media for steel production. Frontiers in Materials. 2022;9:1010156. https://doi.org/10.3389/fmats.2022.1010156
14. Boston Metal: electrolyzing as a pure alternative to manufacturing. Available at URL: https://econet.ru/articles/boston-metal-elektroliz-kakchistaya-alternativa-proizvodstvu-stali (Accessed 10.06.2025). (In Russ.).
15. Mehta A. Meet the green technologies set to transform the geopolitics of steelmaking. Available at URL: https://www.reuters.com/sustainability/decarbonizing-industries/meet-green-technologies-set-transform-geopolitics-steelmaking-2025-05-28/. (Accessed 10.06.2025).
16. Humbert M.S., Brooks G.A., Duffy A.R., etc. Economics of electrowinning iron from ore for green steel production. Journal of Sustainable Metallurgy 2024;10:1679–1701. https://doi.org/10.1007/s40831-024-00878-3
17. Pavlov V.V. Inconsistencies of Metallurgy. Yekaterinburg: USTU; 2013:212. (In Russ.).
18. Roshchin V.E., Kuznetsov Yu.S., Gamov P.A., Salikhov S.P., Smirnov K.I., Suleimenov B., Kosdauletov N., Adilov G., Bil’genov A., Grigor’ev E.V. Production of active metal oxides and concentrates from complex and difficult-to-process iron-bearing ores by selective reduction of elements. Patent RF no. 2826 667C1. SEC C22B5/02; C22B1/00. Bulleten’ izobretenii. 2024:26. (In Russ.).
19. Smirnov K.I., Gamov P.A., Roshchin V.E. Justification of a rational processing scheme for ilmenite concentrate with production of soft iron and titanium dioxide concentrate. CIS Iron and Steel Review. 2025;29:4–10. https://doi.org/10.17580/cisisr.2025.01.01
20. Patisson F., Mirgaux O. Hydrogen ironmaking: How it works. Metals. 2020;10(7):922. https://doi.org/10.3390/met10070922
21. Ma Y., Filho I.R.S., Bai Y., Schenk J., Patisson F., Beck A., van Bokhoven J.A., Willinger M.G., Li K., Xie D., Ponge D., Zaefferer S., Gault B., Mianroodi J.R., Raabe D. Hierarchical nature of hydrogen-based direct reduction of iron oxides. Scripta Materialia. 2022;213:114571. https://doi.org/10.1016/j.scriptamat.2022.114571
22. Cavaliere P. Hydrogen Assisted Direct Reduction of Iron Oxides. Springer Cham; 2002:399. https://doi.org/10.1007/978-3-030-98056-6
23. Lüngen H.B., Schmöle P. The way the European steel industry wants to become carbon neutral. In: Proceedings of 9th European Coke and Ironmaking Congress (ECIC 2024). Coal, Coke, Biocoal, Biocoke, Biochar and Iron Reductions. Italy, Bardolino, 16 – 18 October 2024. Italy: Associazione Italiana di Metallurgia (AIM); 2024:1–12.
24. West K. High-temperature molten oxide electrolysis steelmaking (ULCOLYSIS). Available at URL: https://energy.nl/data/high-temperature-molten-oxide-electrolysis-steelmaking-ulcolysis/ (Accessed 10.06.2025).
25. Electrolysis in ironmaking. Fact sheet. Available at URL: https://worldsteel.org/wp-content/uploads/Fact-sheet-Electrolysis-in-ironmaking.pdf (Accessed 10.06.2025).
26. Primary Exploration of Hydrogen Metallurgy. Zhang J., Li K., Liu Z., Yang T. eds. Springer. Springer: 2024:410.
About the Authors
V. E. RoshchinRussian Federation
Vasilii E. Roshchin, Dr. Sci. (Eng.), Prof., Chief Researcher of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
A. V. Roshchin
Russian Federation
Anton V. Roshchin, Dr. Sci. (Eng.), Assist. Prof., Leading Researcher of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
Review
For citations:
Roshchin V.E., Roshchin A.V. Electrochemistry of reduction processes and prospects for the development of reduction technologies. Izvestiya. Ferrous Metallurgy. 2025;68(4):424-433. https://doi.org/10.17073/0368-0797-2025-4-424-433