Scroll to:
Promising designs of gas analyzers for metallurgy
https://doi.org/10.17073/0368-0797-2025-4-342-348
Abstract
Gas analysis is one of the key methods for assessing the quality of atmospheric air in populated areas, as well as in the work area of production facilities. Atmospheric air monitoring is especially necessary at facilities that have a significant negative impact on the environment, in particular, at ferrous metallurgy enterprises. The peculiarities of the gas analyzers used for air quality monitoring system are their sensitivity and selectivity. To achieve these indicators, a properly selected sensing element is needed: a gas analyzer converter. Synthesized solid solutions of semiconductor binary components, which proved themselves to be good adsorbents, are proposed as materials for the manufacture of converters. In this paper, the authors examined semiconductor systems consisting of ZnTe and CdSe, conditions for synthesis of the solid solutions based on them, and methods for their identification, which allowed the obtained materials to be certified as solid substitution solutions with cubic sphalerite and hexagonal wurtzite structures (depending on the composition). X-ray, micro-, electron-microscopic, and IR spectroscopic studies of solid solutions made it possible to understand the surface structure of adsorbents. Results of the studies of the surface chemical composition, acid-base properties of solid solutions and binary components of the system allow us to conclude that the Lewis and Brønsted acid centers responsible for CO adsorption on the surface are present on the surface. In the ZnTe – CdSe systems, there is a tendency to move from a slightly acidic region to a relative increase in the surface basicity with an increase in ZnTe content. When materials are placed in a CO atmosphere, gas adsorption on the surface of solid solutions occurs in the same dependence, which was confirmed by the direct catalytic studies. The established patterns of changes with the composition of bulk and surface properties allow us to recommend new obtained materials as primary converters of sensors.
Keywords
For citations:
Vasina M.V., Bashchenko L.P. Promising designs of gas analyzers for metallurgy. Izvestiya. Ferrous Metallurgy. 2025;68(4):342-348. https://doi.org/10.17073/0368-0797-2025-4-342-348
Introduction
In industrial zones of metallurgical enterprises, the concentration of carbon monoxide in the ambient air often exceeds permissible limits, posing serious risks to both employee health and the environment. To monitor air quality, gas analyzers are utilized; these devices rely on the bulk and surface properties of their sensing elements, which ensure high sensitivity, rapid response, and selectivity. Timely detection of carbon monoxide in the workplace is critical for preventing accidents, minimizing environmental damage, and safeguarding personnel health.
Semiconductor materials are commonly used as sensing elements in gas analyzers due to their excellent adsorption properties [1]. Their sensitivity is primarily based on gas molecule adsorption at the surface, the formation of space-charge regions, and changes in the concentration of charge carriers in the near-surface layer. The efficiency of adsorption depends on the semiconductor’s structural type, the nature and concentration of active surface centers, and its specific surface area [2]. Gas detection occurs through changes in the electrical conductivity of the sensing element (sensor signal) upon exposure to the target gas [3]. Therefore, the choice of sensing material is a key factor in analyzer performance. One promising approach to enhancing the adsorption capacity of binary materials involves the synthesis of diamond-like semiconductors to produce novel multicomponent materials in the form of solid solutions.
There is growing scientific and practical interest in investigating the previously unexplored physicochemical properties of ZnTe – CdSe solid solutions. These materials demonstrate favorable performance characteristics combined with low production costs, making them promising candidates for sensor applications [4]. Varying the component ratio in the ZnTe – CdSe system allows for the synthesis of solid solutions with tailored properties, enabling their use in a wide range of applications.
The aim of this study is to synthesize and characterize ZnTe – CdSe solid solutions and to determine potential areas of practical application based on their physicochemical properties.
To achieve this, the following objectives were set:
– to synthesize and characterize ZnTe – CdSe solid solutions;
– to investigate the physicochemical surface properties of the system components;
– to evaluate the application potential of the resulting materials in sensor technology as a cost-effective alternative.
Materials and methods
The study investigated fine powders of the binary compounds ZnTe and CdSe, along with their solid solutions (ZnTe)x(CdSe)1 – x , synthesized using a method specifically developed for this material system [5]. The powders had specific surface areas ranging from 0.3 to 0.91 m2/g. Confirmation of successful synthesis, formation of solid solutions, and structural characteristics was carried out through X-ray diffraction (XRD), optical and electron microscopy, and infrared (IR) spectroscopy. The molar compositions of the synthesized solid solutions were verified against elemental compositions derived from scanning electron microscopy (SEM) images.
XRD analysis was performed using a D8 Advance Powder X-ray diffractometer (Bruker AXS, Germany) with CuKα radiation (λ = 0.15406 nm) at Т = 293 K. Measurements followed a wide-angle scanning protocol [6; 7], using a Lynxeye position-sensitive detector. Data interpretation and refinement of the crystal lattice parameters were conducted using the ICDD PDF-2 powder diffraction database and TOPAS 3.0 software (Bruker) [8].
Microscopic examinations were carried out using a KN 8700 microscope (Hilox, Japan) and a Micromed POLAR 3 optical microscope with a resolution capacity of up to 7000× [9]. SEM analysis was performed on a JCM–5700 scanning electron microscope equipped with a JED-2300 energy-dispersive spectroscopy (EDS) attachment [10].
Surface acid–base properties were analyzed using hydrolytic adsorption (to determine the isoelectric point) and non-aqueous conductometric titration [11]. Catalytic properties were evaluated using a non-gradient flow-circulation method, under conditions that minimized the influence of mass and heat transfer. Tests were conducted at temperatures ranging from 298 to 423 K and a pressure of 101.3 kPa. The carrier gas (argon) was circulated at a rate of 22 mL/min, and the pulse volume was set to 5 mL. Gas composition after reaction was assessed by chromatographic analysis.
In the hydrolytic adsorption method, the pH value was determined at which the amphoteric adsorbents (ZnTe, CdSe, and their solid solutions (ZnTe)x(CdSe)1 – x ) released equal and minimal amounts of Н+ and ОН– ions. These materials exhibited distinct isoelectric points corresponding to their minimum solubility. The рНiso values were used to assess the average strength and the ratio of acidic to basic surface centers.
Reproducibility and measurement accuracy were evaluated through parallel experiments, with data analyzed using standard methods of mathematical statistics and quantitative analysis. Numerical processing, error estimation, and the construction and analysis of graphical data were performed using Stat-2, Microsoft Excel, and Origin software.
Results and discussion
Synthesis of the solid solutions was performed in two stages: heating of sealed ampoules from 573 to 1273 K, followed by controlled cooling to 725 K [12; 13]. The synthesis parameters are presented in Table 1.
Table 1. Mode of obtaining solid solutions
|
XRD analysis confirmed the formation of substitutional solid solutions in the ZnTe – CdSe system. In the diffraction patterns of the solid solutions, characteristic peaks were shifted relative to those of the binary compounds, indicating changes in the crystal structure [14]. Zinc telluride and solid solutions with excess ZnTe exhibited a cubic sphalerite-type structure, while cadmium selenide and solid solutions with excess CdSe demonstrated a hexagonal wurtzite-type structure [15].
Lattice parameters (а, с), unit cell volume (Vp ), interplanar spacing (dhkl ), and X-ray density (ρr ) varied smoothly or linearly with composition [16]. The size of the coherent scattering region was estimated using the Scherrer equation.
The elemental distribution within the solid solutions was assessed using scanning electron microscopy (SEM) on a JCM-5700 microscope equipped with a nitrogen-free energy-dispersive X-ray spectrometer.
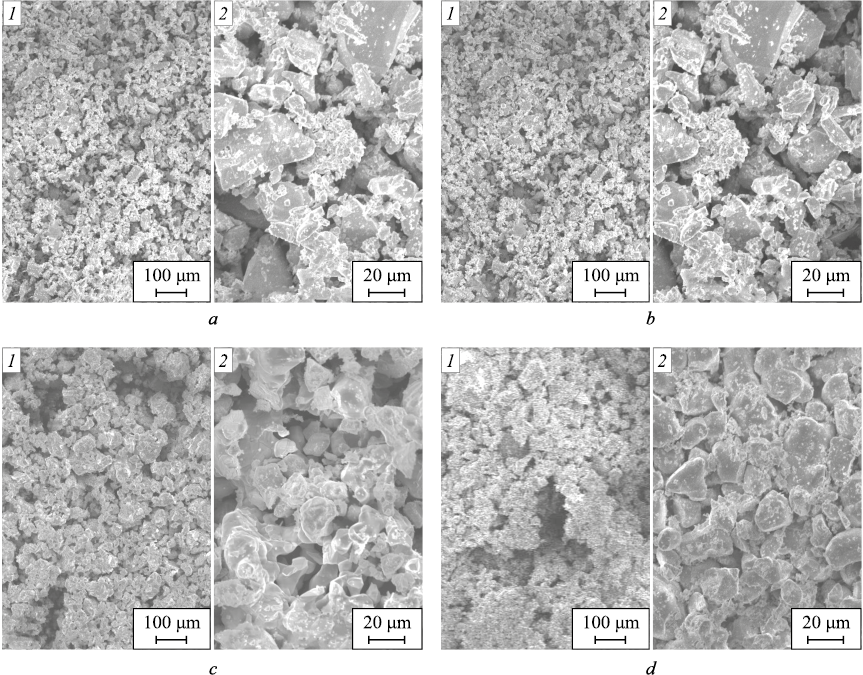
SEM images of the powders of the binary compounds and solid solutions of the studied system are shown in Fig. 1.
Fig. 1. SEM images of powders CdSe (а), (ZnTe)0.26(CdSe)0.74 (b), |
In the phase-contrast SEM images of ZnTe – CdSe solid solutions, bright CdSe inclusions smaller than 5 µm were observed on the homogeneous background of ZnTe grains. ZnTe binary compound is characterized by coarser grains, a feature that persists in the solid solutions with higher ZnTe content (Table 2).
Table 2. Results of particle counting by microscopic analysis
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The specific geometric surface area, surface-area mean diameter, number-average diameter, volume-weighted diameter, and polydispersity index of the systems (Table 3) were calculated using the following formulas:
\[\begin{array}{c}S = \frac{{6\sum {{n_i}d_i^2} }}{{{\rm{\rho }}\sum {{n_i}{d_i}} }} = \frac{6}{{{\rm{\rho }}{d_s}}};\\K = \frac{{{d_n}}}{{{d_q}}},\end{array}\]
where S is the specific geometric surface area, m2/kg; di is the mean diameter of particles in each fraction; n is the number of particles in the system; ρ is the X-ray density of the particles; ds , dn , dq represent the surface-area, number-average, and volume-weighted mean diameters, respectively; K is the polydispersity index.
Table 3. Results of variance analysis
|
The chemical nature of the acid centers responsible for gas adsorption was determined using conductometric titration [17], which also allowed quantification of their concentration on the surface of the ZnTe – CdSe system components. The acid centers responsible for gas adsorption on the surface are as follows: surface atoms with varying degrees of coordination unsaturation – namely, Cd and Zn atoms (Lewis acid centers), as well as adsorbed water molecules and hydroxyl groups ОН– (Brønsted acid centers) [18]. These conclusions were supported by measurements of the isoelectric point (pH) and IR spectra of the surface [19].
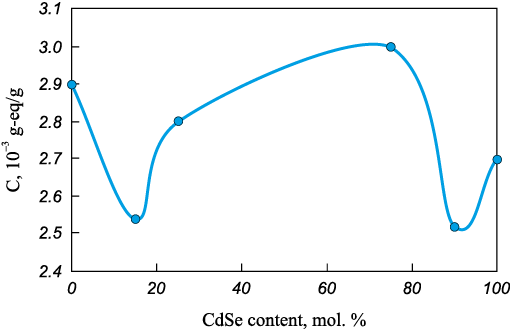
The total concentration of acid centers exhibits an extremal dependence on composition, with maxima observed for (ZnTe)0.26(CdSe)0.74 and (ZnTe)0.68(CdSe)0.32 (Fig. 2). These compositions therefore demonstrate the highest surface acidity, indicating a strong adsorption capacity of (ZnTe)0.26(CdSe)0.74 and (ZnTe)0.68(CdSe)0.32 toward basic gases.
Fig. 2. Dependence of the total concentration of acidic centers |
The increased surface activity (ZnTe)0.68(CdSe)0.32 and (ZnTe)0.26(CdSe)0.74 solid solutions is further supported by the results of acid–base property analysis. The рНiso values of the studied semiconductors (Table 4), measured after air exposure, increase steadily with rising ZnTe content. Upon exposure to CO, extrema appear in the ZnTe – CdSe system corresponding to the compositions (ZnTe)0.68(CdSe)0.32 and (ZnTe)0.26(CdSe)0.74 , and overall, a shift in pH values toward the alkaline region is observed. This behavior is attributed to the high electron density of the carbon and oxygen atoms and the strong double bond between them. The lone electron pairs of CO and its vacant orbitals partially neutralize the coordinatively unsaturated surface atoms (Zn, Cd), thereby enabling the interaction. These findings support the donor–acceptor interaction mechanism [20].
Table 4. рН values of isoelectric state of the surface
| ||||||||||||||||||||||||||||||
The most pronounced difference in рНiso values was observed between CdSe and the solid solutions containing 26 and 68 % ZnTe in CdSe, respectively. These two compositions were selected as candidate sensing materials for carbon monoxide gas analyzers.
The рНiso behavior in a CO atmosphere, along with IR spectroscopic data indicating enhanced CO adsorption in CO + O2 mixtures [19], allows for a preliminary (pre-adsorption testing) prediction of high catalytic activity in (ZnTe)0.26(CdSe)0.74 and (ZnTe)0.68(CdSe)0.32 . This prediction was subsequently confirmed through direct catalytic testing under identical conditions (Fig. 3).
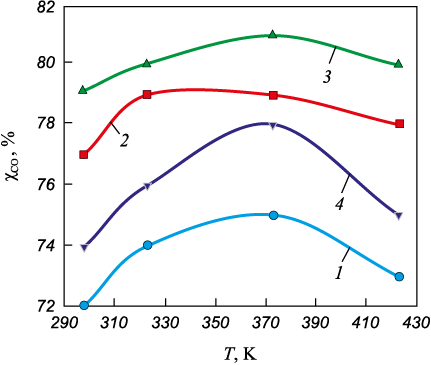
Fig. 3. Dependence of transformation degree (χCO ) |
Analysis of the experimental data indicates a significant degree of catalytic CO conversion (χCO ) even at room temperature. For the (ZnTe)0.26(CdSe)0.74 composition, the CO conversion rate reaches 79 %. As the temperature increases, χCO values generally rise, with maximum conversion observed at 373 K.
A comparison of the CO oxidation efficiency and CO adsorption capacity shows that CdSe and the solid solution (ZnTe)0.26(CdSe)0.74 demonstrate higher catalytic activity, which is consistent with their surface acid–base characteristics. In contrast, ZnTe exhibits lower catalytic activity and adsorption capacity.
In summary, substitutional solid solutions were successfully synthesized from the binary components ZnTe and CdSe. The solid solutions obtained exhibit a cubic structure when zinc telluride is in excess and a hexagonal structure when cadmium selenide predominates. Electron microscopy confirmed the molar and elemental composition of the samples.
Among the synthesized materials the (ZnTe)0.26(CdSe)0.74 and (ZnTe)0.68(CdSe)0.32 compositions demonstrated the largest specific surface area. A similar trend was observed in these samples when analyzing the nature of active surface centers after exposure to air and carbon monoxide, using hydrolytic adsorption and non-aqueous conductometric titration. These results indicate that the surfaces of these solid solutions are highly active toward carbon monoxide. Their enhanced adsorption capacity in both СО and СО + О2 atmospheres was further confirmed by IR spectroscopy.
Conclusions
The study of catalytic CO oxidation over samples of the ZnTe – CdSe system made it possible to preliminarily determine the temperature ranges at which CO oxidation occurs, as well as the most catalytically active compositions. Catalytic tests confirmed the activity of the solid solutions (ZnTe)0.26(CdSe)0.74 and (ZnTe)0.68(CdSe)0.32 , making them suitable for use in environmental diagnostics to detect carbon monoxide in the workplace air of metallurgical enterprises.
References
1. Fang X., Zhai T., Gautam U.K., Li L., Wu L., Bando Y., Golberg D. ZnS nanostructures: From synthesis to applications. Progress in Materials Science. 2011;56(2):175–287. https://doi.org/10.1016/j.pmatsci.2010.10.001
2. Kirovskaya I.A., Nor P.E., Ekkert A.O., Ekkert R.V., Chernous N.V. New materials based on the systems InP–CdTe and CdS–CdTe; Their comparative properties. Inorganic Materials: Applied Research. 2023;14(4):1075–1081. https://doi.org/10.1134/s2075113323040214
3. Peter Y.Yu., Cardona M. Fundamentals of Semiconductors. Physics and Material Properties. Springer; 2010:775. https://doi.org/10.1007/978-3-642-00710-1
4. Kirovskaya I.A., Nor P.E., Bukashkina T.L., Mironova E.V. Surface properties of semiconductor analogs of CdBVI and their solid substitution solutions. Russian Journal of Physical Chemistry A. 2016;90(3):522–529. https://doi.org/10.1134/S0036024416030213
5. Kirovskaya I.A. Physico-Chemical Properties of Binary and Multicomponent Diamond-Like Semiconductors. Novosibirsk: SB RAS; 2015:368. (In Russ.).
6. Smyslov E.F. Express X-ray method for determining the lattice period of nanocrystalline materials. Industrial laboratory. Diagnostics of materials. 2006;72(5):33–34. (In Russ.).
7. Gorelik S.S., Rastorguev L.N., Skakov Yu.A. Radiographic and Electron-Optical Analysis. Moscow: Metallurgiya; 1970:366. (In Russ.).
8. Fedyaeva O.A., Vasina M.V., Poshelyuzhnaya E.G. X-ray powder diffraction and electron microscopic studies of the ZnTe–CdSe system. Russian Journal of Inorganic Chemistry. 2014;59(2):172–175. (In Russ.). https://doi.org/10.7868/S0044457X14020068
9. Clarke A.R., Eberhardt C.N. Microscopy Techniques for Materials Science. Elsevier; 2002:456.
10. Krishtal M.M., Yasnikov I.S., Polunin V.I. Scanning Electron Microscopy and X-ray Spectral Microanalysis in Practical Applications. Moscow: Technosfera; 2009:206. (In Russ.).
11. Kirovskaya I.A. Catalysis. Semiconductor Catalysts. Omsk: OmSTU; 2004:272. (In Russ.).
12. Charbonnier M., Murat M. Sur la détermination des diagrammes de phases à température ambiante des sulfures mixtes appartenant aux systèmes Zn-Cd-S, Zn-Hg-S, Cd-Hg-S. Comptes Rendus de l’Académie des Sciences. 1974;278(4):259–261. (In French).
13. Cherin P., Lind E.L., Davis E.A. The preparation and crystallography of cadmium zinc sulfide solid solutions. Journal of the Electrochemical Society. 1970;117(2):233–236.
14. Kirovskaya I.A., Vasina M.V. Structure and acid-base properties of the surface of semiconductors of the ZnTe–CdSe system. Zhurnal fizicheskoi khimii. 2014;88(10):1569–1576. (In Russ.). https://doi.org/10.7868/S0044453714100227
15. Čapek R.K., Moreels I., Lambert K., De Muynck D., Zhao Q., Tomme A.V., Vanhaecke F., Hens Z. Optical properties of zincblende cadmium selenide quantum dots. Journal of Physical Chemistry C. 2010;114(14):6371–6376. https://doi.org/10.1021/jp1001989
16. Goldstein J.I., Newbury D.E., Echlin P., Joy D.C., Lyman C.E., Lifshin E., Sawyer L., Michael J.R. Scanning Electron Microscopy and X-ray Microanalysis. New York: Plenum Press; 2003:708. https://doi.org/10.1007/978-1-4615-0215-9
17. Kirovskaya I.A., Ekkert R.V., Umansky I.Yu., Ekkert A.O., Kropotin O.V. Comparison of the bulk and surface properties of InBV–ZnS semiconductor solid solutions. Semiconductors. 2020;54(11):1459–1466. https://doi.org/10.1134/S1063782620110147
18. Kirovskaya I.A., Vasina M.V., Mironova E.V., Brueva O.Yu., Ekkert A.O., Zhigarova O.Yu. Relative influence of binary components on the bulk and surface properties of GaAs–CdSe and ZnTe–CdSe solid solutions. Journal of Surface Investigation. X-ray, Synchrotron and Neutron Techniques. 2021;15(2): 321–326. (In Russ.). https://doi.org/10.1134/S1027451021020233
19. Kirovskaya I.A., Vasina M.V., Yuryeva A.V., Shalaeva M.E., Eremin E.N., Matyash Yu.I., Korneev S.A. Chemical composition and acid-base surface properties of solid solutions (ZnTe)x(CdSe)1–x . Omsk Scientific Bulletin. 2014;(1(127)):32–37. (In Russ.).
20. Aven M. Mobility of holes and interaction between acceptor defects in ZnTe. Journal of Applied Physics. 1967; 38(11):4421–4430. https://doi.org/10.1063/1.1709141
About the Authors
M. V. VasinaRussian Federation
Marina V. Vasina, Cand. Sci. (Chem.), Assist. Prof. of the Chair of Industrial Ecology and Safety
11 Mira Str., Omsk 644050, Russian Federation
L. P. Bashchenko
Russian Federation
Lyudmila P. Bashchenko, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Thermal Power and Ecology”
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
Review
For citations:
Vasina M.V., Bashchenko L.P. Promising designs of gas analyzers for metallurgy. Izvestiya. Ferrous Metallurgy. 2025;68(4):342-348. https://doi.org/10.17073/0368-0797-2025-4-342-348