Scroll to:
Application of low-temperature reduction by hydrogen for enhancing the magnetic characteristics of several iron ores
https://doi.org/10.17073/0368-0797-2024-6-644-652
Abstract
The conversion of non-magnetic or weakly magnetic constituents of iron ores into the magnetic ‘magnetite’ phase was investigated using partial reduction by hydrogen at temperatures below 400 °C. The examined four commercial iron ores from Russian and Chinese deposits have significant differences in their compositions and morphologies. All ore samples were crushed using mechanical abrasion in a stamp and sieved with a mesh size of 1.5 mm. Reduction was carried out in a tube furnace under isothermal conditions at 375 and 400 °C for one hour. To study the kinetics of the reduction process, non-isothermal studies of selected ores were сonducted using a thermogravimetric analyzer with heating to 800 °C at a heating rate of 10 °C/min in hydrogen flow. The authors made a detailed characterization of the annealed products using X-ray diffraction, scanning electron microscopy and energy dispersive spectroscopy to determine the magnetic characteristics of initial and partially reduced ores. X-ray diffraction patterns showed hematite peaks in the initial samples; both magnetite and metallic iron peaks were detected in the samples reduced at 375 and 400 °C. Such behavior was observed for all four samples under investigation. The most important result of the study is the confirmation of an order of magnitude increase in saturation magnetization for hematite ores, in addition the reduced ore samples show soft magnetic properties with average coercive force values of approximately 20 kA/m. Application of the low-temperature reduction by hydrogen to iron-containing ores is very promising for production of the materials that could later be subjected to enrichment using magnetic separation methods.
Keywords
For citations:
Konyukhov Yu.V., Khanna R., Maslennikov N.A., Li K., Liang Z., Burmistrov I.N., Karpenkov D.Yu., Kashevskii S.V., Kravchenko M.V. Application of low-temperature reduction by hydrogen for enhancing the magnetic characteristics of several iron ores. Izvestiya. Ferrous Metallurgy. 2024;67(6):644-652. https://doi.org/10.17073/0368-0797-2024-6-644-652
Introduction
Humanity is currently deeply concerned about the issue of global warming, which is primarily linked to carbon dioxide emissions [1; 2]. Approximately 7 % of global CO2 emissions are attributed to the metallurgical industry [3]. On average, producing one ton of crude steel generates 1.9 tons of CO2 emissions [4]. According to the World Steel Association, steel production in the Russian Federation reached 71.5 million tons in 2022, while China produces over 1 billion tons of crude steel annually [5].
Under increasing pressure from environmental groups and society, the metallurgical industry will soon need to reduce its CO2 emissions, necessitating a shift to more environmentally friendly and energy-efficient production technologies. Among the most promising approaches is the direct reduction of iron by hydrogen [6; 7], as this process generates water vapor (H2O) instead of carbon-containing gaseous byproducts (CO/CO2 ).
The widespread adoption of direct reduction by hydrogen is currently limited by the high cost of hydrogen, which depends on the methods used for its production [8]. The main methods for producing hydrogen include steam reforming of methane and natural gas, coal gasification, water electrolysis, pyrolysis, partial oxidation, biotechnology, and atomic hydrogen processes [9]. According to [10], global hydrogen consumption in 2020 was 115 million tons, and forecasts predict this figure will rise to 530 million tons by 2030.
Producing one ton of steel requires approximately 51 kg of hydrogen [11]. Calculations indicate that converting hematite to magnetite consumes only 4.31 kg of hydrogen per ton of Fe3O4 . Therefore, during the initial transition to hydrogen metallurgy, it seems practical to focus on the partial reduction of iron-bearing materials during the beneficiation stage, rather than fully reducing them to metallic iron.
In many iron ores, iron is entirely or partially present as hematite, which is challenging to extract. In such cases, reduction by hydrogen can convert hematite into magnetite, allowing its subsequent extraction through magnetic separation [12; 13]. Magnetite-based super-concentrates, with an iron content exceeding 72 % [14; 15], can be injected into the lower part of blast furnaces [16; 17]. This approach can reduce CO2 emissions by altering the mass and thermal balance of the furnace and eliminating the sintering stage. Additionally, these super-concentrates have potential as the primary raw material for direct iron reduction processes in shaft and hearth furnaces [18]. Using partially reduced oxides as feedstock is expected to shorten the time required for full iron reduction [19], thereby improving production energy efficiency.
Most research on the kinetics of reduction by hydrogen of iron ore materials focuses on processes at temperatures between 500 and 1000 °C, culminating in the production of metallic iron [4; 20]. In these studies, magnetite formation is treated as an intermediate reaction in the overall reduction process leading to pure iron. However, studies on the low-temperature (below 400 °C) reduction of iron ores by hydrogen are rare. This study aimed to investigate the feasibility of converting non-magnetic or weakly magnetic components of iron ores into magnetite through partial reduction in a hydrogen flow at temperatures below 400 °С.
Sample preparation and research methods
The study focused on hematite ore (sinter ore) from the Varichev Mikhailovsky GOK (ore A), iron ore from the Pechegubsky deposit of the Olenegorsk GOK (ore B), and ores provided by partners from China (ores C and D).
For the Russian ores, gangue material was separated using laboratory sieves. All ores were then mechanically ground in a laboratory mortar and sieved through a mesh with 1.5 mm openings.
Isothermal reduction experiments were conducted in a Carbolite Gero KST/KZS tube furnace (UK) at 375 and 400 °C. Ceramic boats with dimensions of 100×20×15 mm were used, and the thickness of the powder layer was maintained at 2 – 3 mm. Samples were preheated in a helium flow, after which the helium flow was replaced with hydrogen. Hydrogen was supplied by SAM-1 and TsvetChrom hydrogen generators (Russia) with a combined capacity of 80 L/h and dried beforehand using a silica gel system. After holding the samples at the target temperature, they were cooled in a helium flow.
Reduction studies under linear heating modes were performed at a rate of 10 °C/min in a hydrogen atmosphere using an SDT Q600 thermogravimetric analyzer (USA).
The phase composition of the samples was analyzed using a TDM-20 tabletop X-ray diffractometer (China) equipped with a copper anode. Diffraction data were processed using Match!3 software (Crystal Impact, Germany).
The gas atmosphere generated during oxidative annealing at 800 °C was studied using a ChemBet Pulsar flow chemisorption analyzer (USA), which also regulated the air flow rate. During the experiments, a U-shaped quartz reactor was heated in an air flow at a rate of 50 °C/min up to 500 °C. The gas-air mixture was then heated further to 800 °C at a rate of 30 °C/min and directed to a Pfeiffer Vacuum OmniStar GSD 320 quadrupole mass spectrometer (Germany). The mass spectrometer analyzed a range of 1 to 300 atomic mass units (amu). Since no significant signals were observed in the ranges of 1 – 10 and 45 – 300 amu, the analysis focused on the range of 10 – 45 amu.
Micrographs were obtained using a TESCAN VEGA3 SB scanning electron microscope (Czech Republic). Elemental analysis was conducted via energy-dispersive spectroscopy (EDS) using an INCA Energy 450 attachment (UK). The probe diameter for elemental composition measurements was 300 nm, with an accuracy ±1 %.
Magnetic properties were measured using a VSM-130 vibrating sample magnetometer (Dexing Magnet Company, China) with a magnetic moment measurement accuracy of 1·10–6 A·m2.
Results and discussion
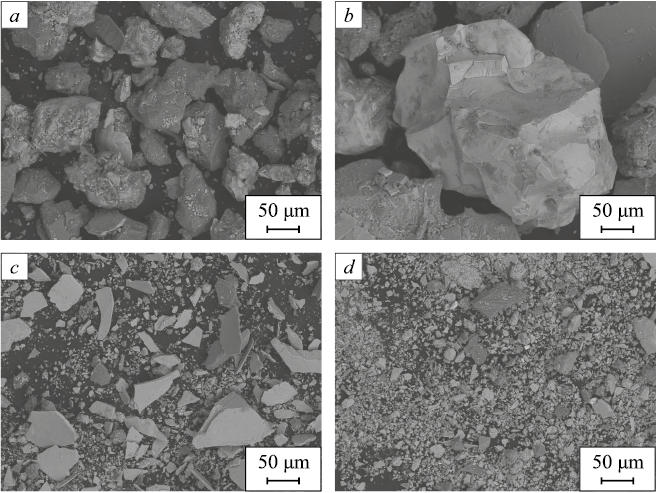
Fig. 1 presents micrographs of the initial materials. The ore particles of Russian origin predominantly have a rounded shape (Fig. 1, a, b), which is typical of natural materials that have not been subjected to intensive grinding. The particle size distribution in ore A is relatively narrow, ranging from 10 to 160 µm. In ore B, most particles are within the range of 50 – 800 µm, with some exceeding 1 mm in diameter. Ore C (of Chinese origin) contains particles with flaky and fractured shapes (Fig. 1, c). The fractured particles are small, ranging from 3 to 35 µm, while the flaky particles are much larger, measuring between 10 and 100 µm in size and 1 – 3 µm in thickness. This microstructure suggests that ore C is like a mixture of two or more types of iron ore materials. Ore D (also of Chinese origin) consists of fractured and spherical particles (Fig. 1, d) of submicron size, ranging from 3 to 35 µm. It is well-known that magnetite ores are generally difficult to grind, and grinding is one of the most cost-intensive operations in mineral beneficiation. The submicron size of the particles in ore D may indicate that the material underwent preliminary conditioning to extract more valuable elements.
Fig. 1. SEM photos: ore A (a), B (b), C (c) and D (d) |
Table 1 summarizes the elemental composition of the initial and hydrogen-treated ores (processed for 1 h at 375 °C), as determined by energy-dispersive X-ray spectroscopy (EDX). The results reveal similar compositions among the studied materials. The Russian ores are characterized by high silicon content (over 20 wt. %), as they had not undergone prior beneficiation. Sodium, at concentrations of 0.7 – 0.8 wt. %, is present only in ore B. Sulfur, in amounts ranging from 0.2 to 0.6 wt. %, is found in all samples except ore C, where no sulfur was detected. The absence of sulfur in ore C may be due to its high calcium content, which could either have been intentionally added or naturally present in the raw material as carbonates or other compounds.
Table 1. Elemental composition of iron ore materials in the initial state
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
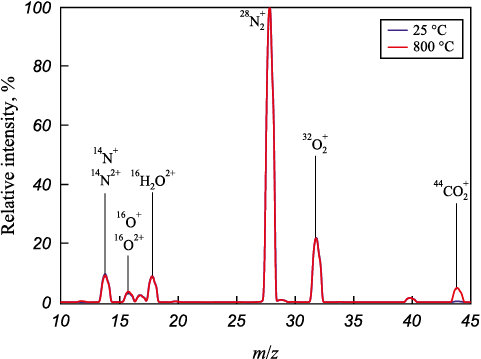
The presence of carbon in the studied iron ore materials was examined by analyzing the gases released during calcination in air. The spectra confirmed that at 800 °C, CO2 is released at m/z = 44, resulting from the decomposition of carbonates. As an example, Fig. 2 shows the mass spectrum of the gas phase generated during the calcination of a sample from ore C in air at 800 °C. The total mass loss during oxidative annealing was 3.52 % for ore A and 3.16 % for ore B. In contrast, the Chinese iron ores exhibited significantly higher mass losses: 19.59 % for ore C and 12.45 % for ore D. These higher values for the Chinese ores can be attributed to their higher carbonate content and lower silica content.
Fig. 2. Mass spectra of the gas phase formed during air blowing |
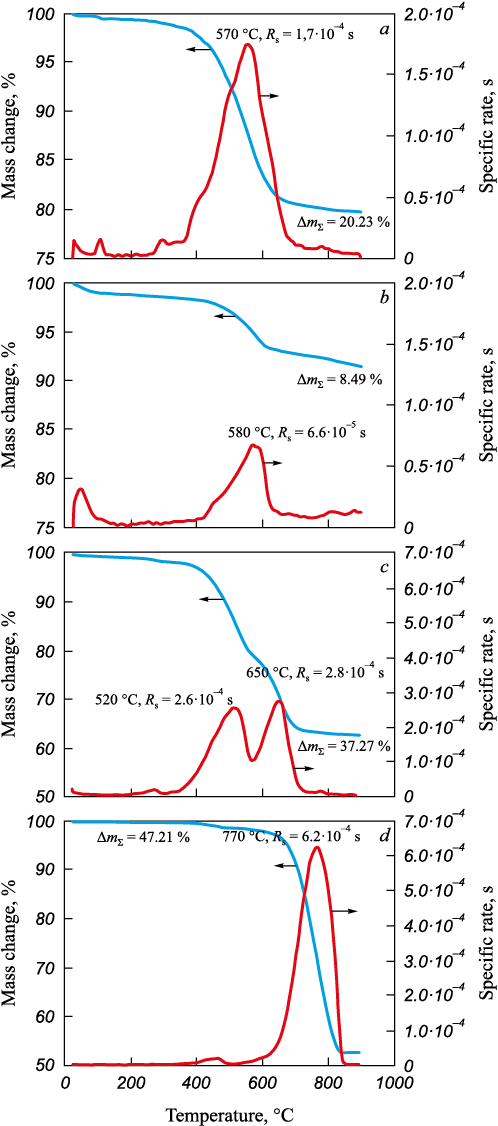
The interaction of iron ore materials with hydrogen under non-isothermal conditions showed noticeable differences in mass-change patterns. A comparative analysis of the thermogravimetric curves (Fig. 3) revealed both common trends and distinctive features in the metallization process of iron ores from different sources in a hydrogen stream. Metallization refers to the partial or complete decomposition of oxides and their reduction to metals or lower oxides.
Fig. 3. Thermogravimetric curves of iron ore processing in hydrogen flow |
For ore A, the first two peaks on the thermogram (Fig. 3, a, DTG curve) correspond to the removal of adsorbed moisture. The peak near 300 °C likely represents the decomposition of hydroxides, which may have formed during the ore’s exposure to moisture. In the temperature range of 350 – 450 °C, hematite is reduced to magnetite. In subsequent stages, magnetite is reduced to metallic iron (below 570 °C), bypassing the formation of wüstite. Since mass loss does not cease at 570 °C, it is possible that an intermediate FeO product forms in the 570 – 800 °C range. A small peak near 800 °C is likely due to the decomposition of carbonates. The maximum reaction rate occurs at 570 °С.
The temperature ranges for the metallization of ores B and A are similar (Fig. 3, b), despite ore B being classified as a magnetite type. Its composition includes small amounts of iron hydroxide and hematite. At the final stage, a significant mass loss occurs due to its higher calcium content and, consequently, a greater amount of carbonates. The maximum reaction rate is observed at 580 °С.
The thermogram of ore C (Fig. 3, c) shows two primary peaks, supporting the earlier hypothesis that this sample was produced by mechanically mixing two different iron ore materials (likely natural ore and a beneficiation byproduct). The maximum reaction rates are observed at 520 and 650 °C. The lower temperature for the first peak can be attributed to the high dispersion of particles, which enhances the reactivity of some of the ore material.
The metallization process for ore D (Fig. 3, d) differs from the other three samples. Despite the high dispersion of this material, reduction begins only at 550 °C, with the maximum reaction rate occurring at 770 °C. This suggests that this iron ore material is not a concentrate but is more likely a byproduct obtained during mineral processing [21].
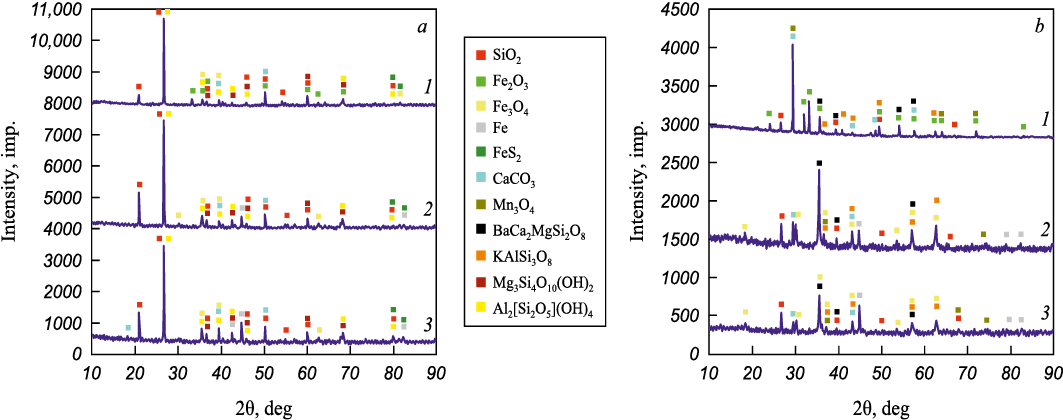
To investigate sample behavior under isothermal conditions, temperatures of 375 and 400 °C were chosen, and samples of ores A and D were processed in hydrogen for 1 h at these temperatures. The diffraction patterns of the initial materials show peaks corresponding to the hematite phase (Fig. 4). Treatment at 375 °C resulted in the complete transformation of the hematite phase into magnetite for all samples. Additionally, peaks for metallic iron appeared in the diffraction patterns, demonstrating the feasibility of producing metallic iron at temperatures below 400 °C. No significant differences were observed between the diffraction patterns obtained at 375 and 400 °C, indicating that the processes at these temperatures are qualitatively similar, differing only slightly in the extent of their completion.
Fig. 4. XRD curves of ore A (a) and ore D (b) in the initial state (1) |
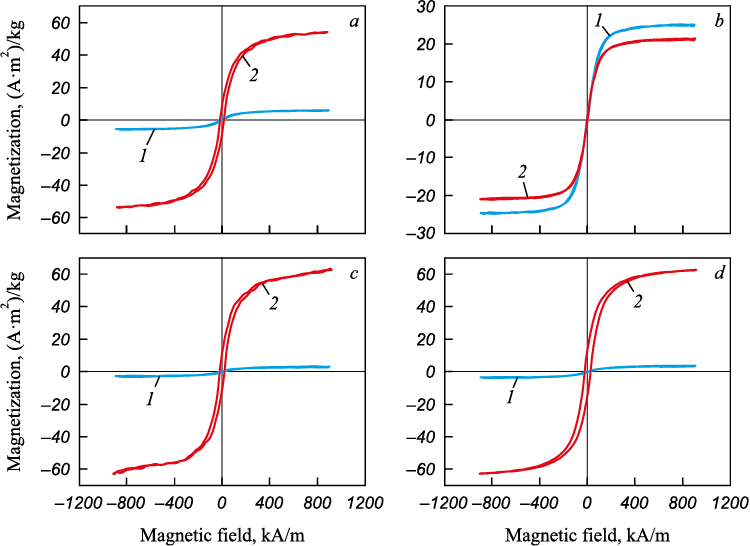
Fig. 5 presents the results of magnetic property measurements for the initial materials and those processed at 375 °C in a hydrogen flow for one hour.
Fig. 5. Hysteresis loops of ore materials in the initial state (1) and processed |
Magnetization measurements were conducted on non-compacted isotropic powders of both the initial ores and the reduction products. The key magnetic characteristics of the samples are summarized in Table 2.
Table 2. Magnetic characteristics for ore materials in the initial state
| ||||||||||||||||||||||||||||||||||||||||||||||||||
An analysis of the field dependence curves indicates that all samples exhibit soft magnetic properties, with average coercive force values around 20 kA/m. The rectangularity coefficient of the hysteresis loops (Mr /Ms ) suggests that the samples are composed of isotropic magnetic phases. Reduction annealing significantly increased the saturation magnetization of all samples (except ore B) by an order of magnitude. This increase is attributed to the formation of numerous iron-containing phases with high magnetic properties during thermal treatment. This observation is supported by X-ray phase analysis results. It is important to note that high saturation magnetization is a critical parameter for optimizing magnetic separation conditions for iron-containing ores.
Thus, the significant enhancement in magnetic properties underscores the potential of low-temperature reduction by hydrogen for producing materials that can be further processed using magnetic separation methods.
Conclusions
The study demonstrated the feasibility of partial reduction of selected types of iron ore materials by hydrogen at temperatures below 400 °C, including those with a high SiO2 content (over 20 wt. %) and large average particle sizes (over 1 mm).
It was established that processing the studied iron ore samples in a hydrogen flow at 375 °C for one hour results in the formation of magnetite phases and partially reduced iron. No residual hematite phases were detected in the diffraction patterns of the samples.
The processing of hematite iron ores with hydrogen at 375 °C significantly enhances their magnetic properties, making the material promising for enrichment using magnetic separation methods.
References
1. Rebonato R., Kainth D., Melin L., O’kane D. Optimal climate policy with negative emissions. International Journal of Theoretical and Applied Finance. 2024;27(01):2450012. https://doi.org/10.1142/S0219024924500122
2. Zou C., Wu S., Yang Z., Pan S., Wang G., Jiang X., Guan M., Yu C., Yu Z., Shen Y. Progress, challenge and significance of building a carbon industry system in the context of carbon neutrality strategy. Petroleum Exploration and Development. 2023;50(1):210–228. https://doi.org/10.1016/S1876-3804(22)60382-3
3. Holappa L. A general vision for reduction of energy consumption and CO2 emissions from the steel industry. Metals. 2020;10(9):1117. https://doi.org/10.3390/met10091117
4. Heidari A., Niknahad N., Lijana M., Fabritius T. A review on the kinetics of iron ore reduction by hydrogen. Materials. 2021;14(24):7540. https://doi.org/10.3390/ma14247540
5. Qiao Y., Wang G. Recent status of production, administration policies, and low-carbon technology development of China’s steel industry. Metals. 2024;14(4):480. https://doi.org/10.3390/met14040480
6. Spreitzer D., Schenk J. Reduction of iron oxides with hydrogen – A review. Steel Research International. 2019;90(10): 1900108. https://doi.org/10.1002/srin.201900108
7. Zhang J., Li K., Liu Z., Yang T. Primary Exploration of Hydrogen Metallurgy. Springer Singapore; 2024:391.
8. Lagioia G., Spinelli M.P., Amicarelli V. Blue and green hydrogen energy to meet European Union decarbonisation objectives. An overview of perspectives and the current state of affairs. International Journal of Hydrogen Energy. 2023;48(4):1304–1322. https://doi.org/10.1016/j.ijhydene.2022.10.044
9. Dash S.K., Chakraborty S., Elangovan D. A brief review of hydrogen production methods and their challenges. Energies. 2023;16(3):1141. https://doi.org/10.3390/en16031141
10. Zhiznin S.Z., Shvets N.N., Timakhov V.M., Gusev A.L. Economics of hydrogen energy of green transition in the world and Russia. Part I. International Journal of Hydrogen Energy. 2023;48(57):21544–21567. https://doi.org/10.1016/j.ijhydene.2023.03.069
11. Kukushkin A.B., Kukushkin A.S., Levashova M.G., Lisitsa V.S., Neverov V.S., Pshenov A.A., Sdvizhenskii P.A., Tolstikhina I.Yu., Khusnutdinov R.I., Serov S.V., Tugarinov S.N. The effect of thermodynamic nonequilibrium of hydrogen recycling on the charge-exchange spectroscopy of tokamak edge plasma. Problems of Atomic Science and Technology. Series: Thermonuclear Fusion. 2022;45(3):12–22.
12. Svoboda J. Magnetic Techniques for the Treatment of Materials. Springer Science & Business Media; 2004:642. https://doi.org/10.1007/1-4020-2107-0
13. Yuan S., Wang R., Zhang Q., Li Y., Gao P. Extraction and phase transformation of iron in fine-grained complex hematite ore by suspension magnetizing roasting and magnetic separation. Korean Journal of Chemical Engineering. 2022; 39:1891–1901. https://doi.org/10.1007/s11814-022-1116-1
14. Zelenova I.M. Iron-ore concentrates in iron-powder production. Steel in Translation. 2009;39(9):827–830 (2009). https://doi.org/10.3103/S0967091209090216
15. Syrkov A.G., Prokopchuk N.R. Dispersed iron obtaining by the method of solid state hydride synthesis and the problem of hydrophobicity of metal. CIS Iron and Steel Review. 2021;21:16–22. https://doi.org/10.17580/cisisr.2021.01.03
16. Mоskalina A.A., Chaika A.L., Kornilov B.V., Lebed’ V.V., Dzhigota M.G. Improvement of blast furnace energy efficiency by injection of preheated pulverized coal fuel with iron oxides. Steel in Translation. 2021;51(7):468–474. https://doi.org/10.3103/S0967091221070056
17. Borodulin A.V., Chaika A.L., Sokhatskii A.A., Kornilov B.V. Investigation of impact of injection of pulverized ferric oxides and preheated pulverized-coal fuel in hearth to indexes of heat operation of blast furnace. Ecologiya i promyshlennost’. 2016;(2(47)):48–53. (In Russ.).
18. Yusfin Y.S., Pashkov N.F. Metallurgy of Iron. Moscow: Akademkniga; 2007:464.
19. Bogdandy L., Engell H.-J. Die Reduktion der Eisenerze. Düsseldorf: Springer Verlag; 1967:539. (In Germ.).
20. He J., Li K., Zhang J., Conejo A.N. Reduction kinetics of compact hematite with hydrogen from 600 to 1050 °C. Metals. 2023;13(3):464. https://doi.org/10.3390/met13030464
21. Lileev A.S., Konyukhov Yu.V., Zhukov D.G., Khanna R. Properties of nanocrystalline magnetic powders of the Fe–O system Obtained from iron ore dust using magnetic pulse processing. Inorganic Materials: Applied Research. 2024; 15(3):883–888. https://doi.org/10.1134/S2075113324700333
About the Authors
Yu. V. KonyukhovRussian Federation
Yurii V. Konyukhov, Dr. Sci. (Eng.), Prof., Head of the Chair of Mineral Processing and Industrial Wastes Recycling
4/1 Leninskii Ave., Moscow 119049, Russian Federation
R. Khanna
Australia
Rita Khanna, PhD, Prof.
Sydney, NSW 2052, Australia
N. A. Maslennikov
Russian Federation
Nikita A. Maslennikov, Assistant of the Chair of Mineral Processing and Industrial Wastes Recycling
4/1 Leninskii Ave., Moscow 119049, Russian Federation
K. Li
China
Kejiang Li, PhD, Prof.
Beijing 100083, China
Z. Liang
China
Zeng Liang, PhD Student
Beijing 100083, China
I. N. Burmistrov
Russian Federation
Igor’ N. Burmistrov, Dr. Sci. (Eng.), Leading Expert of the Chair of Functional Nanosystems and High-Temperature Materials
4/1 Leninskii Ave., Moscow 119049, Russian Federation
D. Yu. Karpenkov
Russian Federation
Dmitrii Yu. Karpenkov, Cand. Sci. (Phys.-Math.), Senior Researcher of the Chair of Magnetism
1 Leninskie Gory, Moscow 119991, Russian Federation
S. V. Kashevskii
Russian Federation
Sergei V. Kashevskii, MA Student of the Chair of Functional Nanosystems and High-Temperature Materials
4/1 Leninskii Ave., Moscow 119049, Russian Federation
M. V. Kravchenko
Russian Federation
Maksim V. Kravchenko, Cand. Sci. (Eng.), Director of Branch in Tashkent
14 Krasnokazarmennaya Str., Moscow 119049, Russian Federation
Review
For citations:
Konyukhov Yu.V., Khanna R., Maslennikov N.A., Li K., Liang Z., Burmistrov I.N., Karpenkov D.Yu., Kashevskii S.V., Kravchenko M.V. Application of low-temperature reduction by hydrogen for enhancing the magnetic characteristics of several iron ores. Izvestiya. Ferrous Metallurgy. 2024;67(6):644-652. https://doi.org/10.17073/0368-0797-2024-6-644-652