Scroll to:
Determination of hydrogen influence on microhardness and microstructure characteristics of aviation alloys
https://doi.org/10.17073/0368-0797-2024-3-332-339
Abstract
This paper presents results of the studies of hydrogen exposure duration influence on the characteristics of two aviation alloys at atmospheric pressure and room temperature. First alloy (alloy 1) was obtained by hot isostatic pressing, and was used for the manufacture of gas turbine rotor discs. Second alloy (alloy 2) was obtained by directional crystallization, and was used for the manufacture of gas turbine blades. It was determined that microhardness of the samples increased during 1000 h of hydrogen exposure duration. The relative increase of the microhardness was insignificant, and for the sample of alloy 1 it was 2.5 %, and for the sample of alloy 2 – 2 %. Correlation analysis of the XRD diagram parameters indicated positive and negative statistically significant relationships correlation between XRD diagrams peaks parameters, hydrogen exposure duration and microhardness of the samples. It was revealed that XRD diagrams peaks of alloy 1 were broadened and their heights increased during hydrogenation, which can be associated with a decrease of dislocations in the grains and their local accumulation at the grains boundaries. Conterwise, XRD diagrams peaks of alloy 2 were narrowed, which can indicate an increase of dislocations in the material grain structure. XRD diagrams processing demonstrated that the crystallite size and dislocation density for alloy 1 decreased with a delay from the hydrogenation start, but for alloy 2 these parameters monotonically increased, and it corresponds to microhardness changes trends of the samples during hydrogenation.
Keywords
For citations:
Saulin D.V., Kuzminykh K.G., Poilov V.Z. Determination of hydrogen influence on microhardness and microstructure characteristics of aviation alloys. Izvestiya. Ferrous Metallurgy. 2024;67(3):332-339. https://doi.org/10.17073/0368-0797-2024-3-332-339
Introduction
One of corrosion types accompanied by the destruction of metals and alloys is hydrogen corrosion. Its characteristic feature is that products of interaction between hydrogen and alloy elements, gas phase or alloy structure defects form inside the alloy causing microcracks. The stronger and harder the alloy, the more pronounced is the issue of hydrogen embrittlement, and a hydrogen concentration of a few ppmw in the material is often sufficient to seriously alter the material properties [1].
Hydrogen embrittlement is known to be a process leading to reduced metal viscosity and plasticity caused by the presence of atomic hydrogen. For hydrogen embrittlement to start within the metal structure, hydrogen should diffuse inside it. As known, the rate of hydrogen diffusion in metals depends on concentration of the diffusing agent, temperature, pressure, and crystal structure1 [2]. For example, in body-centered cubic (BCC) lattices of metals, the hydrogen diffusion coefficient is usually four to five orders of magnitude higher than in face-centered cubic (FCC) lattices or hexagonal close-packed (HCP) ones. However, there are exceptions, such as Pd (FCC) and Co (HCP) metals, which have diffusion coefficient values several orders of magnitude larger than most other metals with BCC and HCP lattices.
If we exclude the processes of hydride formation or hydrogen interaction with carbides, the hydrogen saturation of alloys is usually divided into types related to the features and mechanisms of hydrogen interaction with the metal crystal lattice and its grains, which help to explain the features of hydrogen embrittlement processes. These mechanisms form the basis of the best-known micromechanical models of hydrogen-material interactions: HEDE, HELP, AIDE and HESIV [2 – 5]. There are also combined hydrogen embrittlement models, however, most researchers opt for HEDE and HELP. Thus, the authors of [6 – 8] note that HELP (hydrogen-enhanced localized plasticity) mechanism is likely to proceed simultaneously with HEDE (hydrogen-enhanced decohesion), i.e., hydrogen causes hardening and softening of the material at the same time. Meanwhile, quantitative measurement of the local distribution of hydrogen concentrations in alloys is a serious challenge yet to be solved, which hampers researchers to fully verify the models including hydrogen diffusion.
According to findings of the study presented in [9], the relationship between plasticity and hydrogen-induced fracture mechanism, in addition to changing plasticity and accelerating evolution of metal microstructure, also leads to local high concentrations of hydrogen and a local stress state. The conditions under which cracks emerge due to hydrogen embrittlement are determined by dislocation processes enhanced and accelerated in the presence of hydrogen.
The theory of “hydrogen traps” is also intriguing. Thus, the authors of [10] describe the interaction of hydrogen with defects in the crystal lattice, classify hydrogen traps into reversible, irreversible, and mixed based on their energy levels and demonstrate the impact of hydrogen traps on the hydrogen diffusion coefficient. Regarding diffusive mobility of hydrogen in steel, the authors of [11] investigated the impact of diffusively mobile hydrogen on the plasticity of aircraft steel intended for power parts and assemblies of aviation equipment. The authors note that it is not the total hydrogen content in the metal that determines hydrogen embrittlement. Steel plasticity considerably reduces due to diffusion-mobile hydrogen only, as it has low binding energy with defects in the crystal lattice and gradually moves to the zone of maximum stresses.
As to changes in the metal microstructure in the presence of hydrogen, the paper [12] discusses the HEDE and HELP mechanisms of fatigue crack formation. Moreover, the HELP mechanism considers that hydrogen facilitates movement of dislocations (defects in the crystal lattice) inside the metal grains. In this case, dislocations can accumulate both inside the metal grains and at the grain boundaries, resulting in changing width of XRD diagrams peaks. The broadening XRD diagrams peaks will indicate a more uniform distribution of dislocations (defects) across the grains. On the contrary, when XRD diagrams peaks narrow, the number of dislocations (defects) in the grains will decrease, at the same time, the accumulation of dislocations at the grain boundary may be observed. The Scherrer formula can be used to calculate the dependence of the crystallite size on the variation of the XRD diagrams peaks width [12; 13] and the Williamson-Hall method – to the value of the average relative lattice deformation and dislocation density [14 – 16].
Due to the fact that in the presence of hydrogen, defects in the metal structure can sooner or later lead to cracks and destruction of the metal, the main objective of the work is to determine the effect of a hydrogen atmosphere on the microstructure of aircraft alloys at room temperature and atmospheric pressure.
Characteristics of initial materials
The samples of aircraft alloys, which are widely used to manufacture gas turbines, served as initials materials:
– sample of alloy 1 containing Ni, Co, Cr, Al, Ti, Mo, Nb, W, similar to VV750P alloy described in the [17], obtained by hot isostatic pressing and used to manufacture gas turbine rotor disks, for example, the PD-14 engine.
– sample of alloy 2 containing Ni, Al, Co, Cr, W, Ta, Re, similar to alloy ZhS32 described in [18], obtained by the method of directional crystallization and used to manufacture gas turbine blades.
Hydrogen used for hydrogenation of the samples was obtained using a TsvetChrome-50AV hydrogen generator.
Methods of the experiment and analysis, study parameters
The effect of hydrogen on alloy samples was studied at room temperature and atmospheric pressure. The alloy samples were placed in a sealed glass container filled with pure hydrogen obtained in the hydrogen generator and held at room temperature for a given time, their characteristics (microhardness and phase composition) were periodically monitored. The samples were held in hydrogen medium for more than 1000 h.
The Vickers hardness of the samples was measured using a Q60N, Qness hardness tester with a load of 9.807 N (1 kgf). As microhardness of the samples’ surface is not heterogeneous, all periodic measurements of microhardness in the course of hydrogenation were performed in the zones of previous measurements, and there were minimum 12 such zones. The measurement results were then processed, anomalous values were discarded and the average value of the sample surface microhardness was determined.
The crystal structure of the alloys was investigated using XRD7000 X-ray diffractometer, Shimadzu (CuKα radiation, λ = 1.5406 Å). The XRD diagrams were recorded when the samples were rotating, at tube voltage of 30 kV, current of 30 mA, scanning speed of 1°/min with a step of 0.02°. The XRD diagrams were processed using XRD 6000/7000 Ver. 5.21 software.
Results and discussion
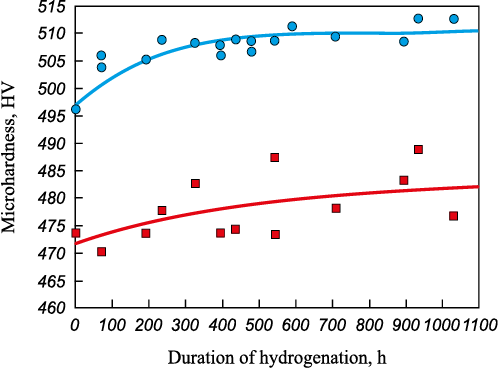
Fig. 1 shows the changing microhardness of the samples during hydrogenation at room temperature. We can see that the average microhardness of the sample of alloy 1 is higher than that of the sample of alloy 2. In this case, during hydrogenation microhardness in the samples of both alloys 1 and 2 slightly increases and it changes the most during the first 400 – 500 h. During 1000 h of hydrogen exposure at room temperature, the microhardness in the sample of alloy 1 changed by about 2.5 %, and that in the sample of alloy 2 – by 2 %. It should be noted that the dispersion of microhardness values in both cases is very large.
Fig. 1. Change in microhardness of the samples during hydrogenation at room temperature: |
We conducted correlation analysis to test the hypothesis that microhardness of alloy samples depends on the duration of the hydrogenation process. The coefficients of correlation between the samples microhardness and duration of hydrogenation were calculated in MS Excel. The calculation showed a positive correlation between the process duration and the samples microhardness. It was found that the correlation coefficient is 0.775 at Rcr = 0.482 for alloy 1 and 0.556 at Rcr = 0.553 for alloy 2, that is correlation coefficients are statistically significant.
According to the XRD diagrams, the sample of alloy 1 has a cubic structure Pm-3m and contains four main (by decreasing intensity) peaks: 43.60 (hkl = 111), 50.50 (hkl = 200), 74.60 (hkl = 220) and 90.40 (hkl = 311), while the sample of alloy 2 has a close-packed cubic face-centered structure Fm-3m (cubooctahedron) and contains five main (by decreasing intensity) peaks: 43.60 (hkl = 111), 50.60 (hkl = 200), 40.60 (hkl = 110), 90.40 (hkl = 311) and 74.60 (hkl = 220).
To determine the influence of hydrogenation on the structure of alloys, we conducted a correlation analysis of the XRD diagram parameters recorded for alloy samples at different durations of hydrogen exposure. As XRD diagrams feature different number of peaks, for the correlation analysis, we selected three of them with the same hkl index: 111, 200 and 311.
We used the following values as XRD diagram parameters for the correlation analysis:
– lattice spacing (d), Å;
– peak intensity (I), imp.;
– peak full width at half maximum (FWHW), deg;
– integral intensity or peak area (S), impulses per degree;
– duration of sample exposure to hydrogen (τ), h;
– values of current average Vickers microhardness of the sample, HV.
Table 1 presents the results of the correlation analysis for the sample of alloy 1. The correlation coefficients in absolute value exceeding the critical correlation coefficient (Rcr = 0.621), i.e., statistically significant ones, are highlighted in bold and underlined.
Table 1. Correlation coefficients of XRD diagram parameters for alloy 1
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The analysis of the calculation results shows that for all peaks there is a negative correlation between the hydrogenation duration and the peaks width, i.e., in the course of hydrogenation, all peaks narrow. We should also note the negative correlation between intensity of the peak with hkl: 200 and its width and positive correlation between the peak intensity and the process duration or hardness.
Thus, the correlation analysis shows that as the hydrogenation duration increases, so does the microhardness in the sample of alloy 1, while the peaks narrow, which can be interpreted as a decrease in the number of defects in the material grain structure or the local arrangement of dislocations, for example, at the grain boundary, which can subsequently lead to the structure fracture along the grain boundaries [19; 20].
Table 2 presents the results of the correlation analysis for the sample of alloy 2. The correlation coefficients in absolute value exceeding the critical correlation coefficient (Rcr = 0.669), i.e., statistically significant ones, are highlighted in bold and underlined.
Table 2. Correlation coefficients of XRD diagram parameters for alloy 2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The calculation results show that alloy 2, unlike alloy 1, features a positive correlation between the duration of hydrogen exposure and the width of the peak with hkl: 111, but negative correlation between the exposure duration and peak intensity, i.e., with increasing duration of hydrogen exposure, the peak widens and its intensity drops. The peaks with hkl: 200 and 311 also demonstrate a negative correlation between the peaks’ width and their intensity. There is a statistically significant correlation between microhardness and XRD diagram parameters only for the peak with hkl: 311. With increasing microhardness, the intensity of this peak drops and the peak area expands. In addition, the peak with hkl: 200 of alloy 2 shows a negative correlation between the lattice spacing value and the peak width, which was not the case for alloy 1.
Thus, with increasing duration of hydrogen exposure, the microhardness of the sample enhances, but the intensity of some peaks drops (hkl: 111 and 311) when these peaks show a significant negative correlation between the peak width and its intensity. The peak with hkl: 111, in contrast to the peak with the same hkl of alloy 1, features a positive correlation between the process duration and the peak width, which can be interpreted as an increase in the number of dislocations (defects) in the material grain structure.
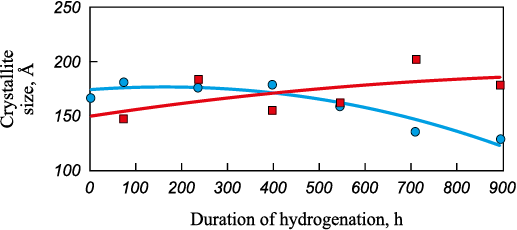
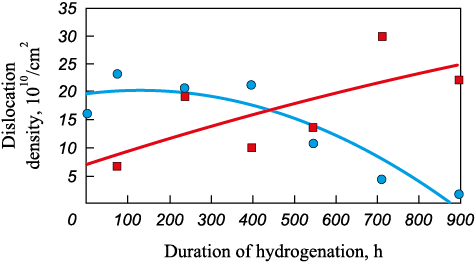
We used the Scherrer formula to calculate the crystallite size and the Williamson-Hall method to determine the lattice characteristics and dislocation density from the XRD diagram parameters. Figs. 2 and 3 demonstrate the results of calculating the change in the average crystallite size and dislocation density during hydrogenation.
Fig. 2. Effect of hydrogen exposure duration on crystallite size:
Fig. 3. Effect of hydrogen exposure duration on dislocation density: |
The graphs show that the average crystallite size of alloy 1 shrinks (by more than 30 %) during the process, while that of alloy 2 increases (by less than 25 %). Meanwhile, the crystallite size of alloy 1 remains almost constant during 400 h of hydrogen exposure and then begins to reduce, while the crystallite size of alloy 2 increases. Similar dependencies are observed for the change in dislocation density (Fig. 3). The dislocation density of alloy 1 drops to almost zero values with a delay of 400 h, while for alloy 2, this value increases.
According to the graphs in Fig. 3, microhardness in the sample of alloy 1 obtained by hot isostatic pressing mostly changes during the first 400 – 500 h, while subsequent changes are very slight. Thus, it can be concluded that exposure of alloy 1 to hydrogen for 400 – 500 h at room temperature and atmospheric pressure leads to hydrogen accumulation in the sample of alloy 1, its microhardness enhancing, while its the microstructure remaining the same. At further saturation of the sample with hydrogen, the alloy microstructure alters practically without any changes in its hardness. For alloy 2 obtained by directional crystallization, the metal hardness and microstructure change continuously when exposed to hydrogen.
Conclusions
The investigation revealed that as alloy samples are exposed to hydrogen for 1000 h, the samples microhardness increases, its relative growth in the sample of alloy 1 reaching 2.5 %, and in the sample of alloy 2 amounting to 2 %. The correlation analysis of the change in XRD diagram parameters during hydrogenation of alloy samples indicated positive and negative statistically significant relationships correlation between XRD diagram peak parameters, hydrogen exposure duration and microhardness of the samples. It was found that alloy 1, being exposed to hydrogen, features narrowing and lengthening of XRD diagram peaks, which may indicate a decrease in the number of dislocations (defects) in the grains or their local accumulation at the grain boundaries of the material. On the contrary, alloy 2, being exposed to hydrogen, features some widening of XRD diagram peaks, which can be indicative of increased dislocations in the material grain structure. The calculations of the effective crystallite size and average dislocation density indicated that during hydrogenation.There was a delayed decrease in both crystallite size and dislocation density for alloy 1. In contrast, these parameters increased monotonically for alloy 2. These findings align with the observed trends in microhardness changes during hydrogenation for both alloys.
References
1. Sun B., Lu W., Gault B., Ding R., Makineni S.K., Wan D., Wu C.-H., Chen H., Ponge D., Raabe D. Chemical heterogeneity enhances hydrogen resistance in high-strength steels. Nature Materials. 2021;20:1629–1634. https://doi.org/10.1038/s41563-021-01050-y
2. Gonzalez M.S., Hernandez I.R. Review: Hydrogen embrittlement of metals and alloys in combustion engines. Tecnología en Marcha. 2018;31(2):3–13. http://dx.doi.org/10.18845/tm.v31i2.3620
3. Djukic M.B., Zeravcic V.S., Bakic G.M., Sedmak A., Rajicic B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Engineering Failure Analysis. 2015;58–2:485–498. https://doi.org/10.1016/j.engfailanal.2015.05.017
4. Traidia A., Chatzidouros E., Jouiad M. Review of hydrogen-assisted cracking models for application to service lifetime prediction and challenges in the oil and gas industry. Corrosion Reviews. 2018;36(4):323–347. https://doi.org/10.1515/corrrev-2017-0079
5. Bruck S., Schippl V., Schwarz M., Christ H.-J., Fritzen C.-P., Weihe S. Hydrogen embrittlement mechanism in fatigue behavior of austenitic and martensitic stainless steels. Metals. 2018;8(5):339. https://doi.org/10.3390/met8050339
6. Djukic M.B., Bakic G.M., Zeravcic V.S., Sedmak A., Rajicic B. Review: The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Engineering Fracture Mechanics. 2019;216:106528. https://doi.org/10.1016/j.engfracmech.2019.106528
7. Sergeev N.N., Sergeev A.N., Kutepov S.N., Gvozdev A.E., Ageev E.V. A review of theoretical concepts of hydrogen cracking in metals and alloys. Proceedings of the Southwest State University. 2017;21(3):6–33. (In Russ.). https://doi.org/10.21869/2223-1560-2017-21-3-6-33
8. Yakovlev Yu.A., Polyanskii V.A., Sedova Yu.S., Belyaev A.K. Models of hydrogen influence on the mechanical properties of metals and alloys. PNRPU Mechanics Bulletin. 2020;(3):136–160. (In Russ.). https://doi.org/10.15593/perm.mech/2020.3.13
9. Robertson I.M., Sofronis P., Nagao A., Martin M.L., Wang S., Gross D.W., Nygren K.E. Hydrogen embrittlement understood. Metallurgical and Materials Transactions A. 2015;46:2323–2341. https://doi.org/10.1007/s11661-015-2836-1
10. Sergeev N.N., Kutepov S.N. On the interaction of hydrogen with lattice defects in metals and alloys. Izvestiya Tul’skogo gosudarstvennogo universiteta. Tekhnicheskie nauki. 2017;(4):131–141. (In Russ.).
11. Voznesenskaya N.M., Tonysheva O.A., Leonov A.V., Dul’nev K.V. Hydrogen influence on high-strength corrosion-resistant steel VNS65-Sh properties and ways of elimination of hydrogen embrittlement. Proceedings of VIAM. 2018;(10):3–9. (In Russ.). https://doi.org/10.18577/2307-6046-2018-0-10-3-9
12. Connolly M., Martin M., Bradley P., Lauria D., Slifka A., Amaro R., Looney C., Park J.-S. In situ high energy X-ray diffraction measurement of strain and dislocation density ahead of crack tips grown in hydrogen. Acta Materialia. 2019;180:272–286. https://doi.org/10.1016/j.actamat.2019.09.020
13. Chaki S.H., Malek T.J., Chaudhary M.D., Tailor J.P., Deshpande M.P. Magnetite Fe3O4 nanoparticles synthesis by wet chemical reduction and their characterization. Advances in Natural Sciences: Nanoscience and Nanotechnology. 2015;6(3):035009. https://doi.org/10.1088/2043-6262/6/3/035009
14. Pushkarev S.S., Grekhov M.M., Zenchenko N.V. X-Ray diffraction analysis of features of the crystal structure of GaN/Al0.32Ga0.68N HEMT-heterostructures by the Williamson–Hall method. Semiconductors. 2018;52(6):734–738. https://doi.org/10.1134/s1063782618060209
15. Wang L., Cheng X., Peng H., Zhao P.W., Cai Z.X. Effect of tempering temperature on hydrogen embrittlement in V-containing low alloy high strength steel. Materials Letters. 2021;302:130327. https://doi.org/10.1016/j.matlet.2021.130327
16. Mote V., Purushotham Y., Dole B. Williamson-Hall analysis in estimation of lattice strain in nanometer-sized ZnO particles. Journal of Theoretical and Applied Physics. 2012;6:6. https://doi.org/10.1186/2251-7235-6-6
17. Volkov A.M., Vostrikov A.V., Bakradze M.M. Development principles and alloying features of P/M Ni-base superalloys for jet-engine disks application. Proceedings of VIAM. 2016;(8):2. (In Russ.). http://dx.doi.org/ 10.18577/2307-6046-2016-0-8-2-2
18. Kolyadov E.V., Rassokhina L.I., Visik E.M., Gerasimov V.V., Filonova E.V. Study of single crystal turbine blades made of ZhS32 alloy with a promising scheme of cooling. Zavodskaya laboratoriya. Diagnostika materialov. 2018;84(10): 35–40. (In Russ.). https://doi.org/10.26896/1028-6861-2018-84-10-35-40
19. Nagao A., Dadfarnia M., Somerday B.P., Sofronis P., Ritchie R.O. Hydrogen-enhanced-plasticity mediated decohesion for hydrogen-induced intergranular and “quasi-cleavage” fracture of lath martensitic steels. Journal of the Mechanics and Physics of Solids. 2018;112:403–430. https://doi.org/10.1016/j.jmps.2017.12.016
20. Nagao A., Smith C.D., Dadfarnia V, Sofronis P., Robertson I.M. The role of hydrogen in hydrogen embrittlement fracture of lath martensitic steel. Acta Materialia. 2012;60(13–14):5182–5189. https://doi.org/10.1016/j.actamat.2012.06.040
About the Authors
D. V. SaulinRussian Federation
Dmitrii V. Saulin, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Chemical Engineering”
29 Komsomolskii Ave., Perm 614990, Russian Federation
K. G. Kuzminykh
Russian Federation
Konstantin G. Kuzminykh, Senior Lecturer of the Chair “Chemical Engineering”
29 Komsomolskii Ave., Perm 614990, Russian Federation
V. Z. Poilov
Russian Federation
Vladimir Z. Poilov, Dr. Sci. (Eng.), Prof. of the Chair “Chemical Engineering”, Head of CUC “Center for High-tech Chemical Technologies and Physico-Chemical Research”
29 Komsomolskii Ave., Perm 614990, Russian Federation
Review
For citations:
Saulin D.V., Kuzminykh K.G., Poilov V.Z. Determination of hydrogen influence on microhardness and microstructure characteristics of aviation alloys. Izvestiya. Ferrous Metallurgy. 2024;67(3):332-339. https://doi.org/10.17073/0368-0797-2024-3-332-339