Scroll to:
Rational application of high quality manganese concentrate
https://doi.org/10.17073/0368-0797-2024-2-237-244
Abstract
The article presents the results of theoretical and experimental studies of manganese reduction processes from oxides of high-quality manganese concentrate obtained by hydrometallurgical enrichment of ferromanganese ores, as well as, from marokite (product of thermal synthesis of concentrate and dolomite) with carbon and silicon. The method of thermodynamic modeling with TERRA software complex determined the optimal temperatures and consumption of reducing agents that ensure the complete reduction of manganese. It was found that any of the above-mentioned reducing agents, or a combination thereof in certain ratios, can be utilized as a reducing agent when using oxide manganese-containing materials for steel treatment. The results of experimental studies made it possible to develop technology for the production of marokite-manganite concentrate and monophase synthetic material (CaMnO3 ). They can be obtained using the technology developed by the authors, which includes mechanical and thermal treatment of a mixture of high-quality manganese concentrate and calcined dolomite or lime. Marokite-manganite concentrate is useful for alloying steel with manganese when it is smelted in an electric furnace or in a ladle furnace unit, and a monophasic synthetic material is efficient for the production of metal manganese. Based on the results of thermodynamic calculations and experimental studies, technological parameters for processing steel with marokite-manganite concentrate in an electric furnace and a ladle furnace unit are proposed. Monophasic synthetic material CaMnO3 should be used as the charge component for the production of metal manganese by the out-of-furnace aluminum thermal treatment, which will increase the thermality of the process, as well as the extraction of manganese at the level of 90 %. The results of experimental studies were obtained using modern research methods with laboratory and analytical equipment, as well as statistical processing methods.
Keywords
For citations:
Rybenko I.A., Rozhikhina I.D., Nokhrina O.I., Golodova M.A. Rational application of high quality manganese concentrate. Izvestiya. Ferrous Metallurgy. 2024;67(2):237-244. https://doi.org/10.17073/0368-0797-2024-2-237-244
Relevance
To supply the metallurgical industry with manganese, a strategically important raw material, state-of-the-art technological developments should be employed for the industrial mining and enrichment of manganese ores from Russian deposits. Russia has significant reserves of manganese ores (more than 290 million tons), but most are of low quality and classified as refractory. These ores have a low manganese content (18 – 24 %), a high specific phosphorus content (P/Mn ratio > 0.006), and increased iron and silicon content [1 ‒ 3].
Significant reserves of ferromanganese ores are concentrated in the Selezenskoye and Kaigadatskoye (32.7 million tons) deposits. Currently, these ores are not utilized in metallurgical production because metallurgical enrichment cannot be applied to them [1; 3; 4].
Over the last two decades, to ensure cost-effective resource use, Russian [5 – 8] and foreign researchers [9 – 12] have been actively exploring new chemical and hydrometallurgical methods for enriching low-grade manganese ores, slags, and sludges [13 – 15]. Nowadays, environmental safety has been added to the existing requirements related to the economic efficiency of the processes [16 – 18].
We conducted thermodynamic calculations and experimental studies on the enrichment of ferromanganese ores in the Kemerovo region (Kuzbass). The findings allowed us to determine the main technological parameters for extracting manganese and iron, and to develop an enrichment process diagram. This enables the production of high-quality concentrates of manganese and iron, with manganese recovery reaching 90 – 92 % and iron recovery amounting to 86 ‒ 90 % [19].
The relevant objectives include studying the processes of manganese reduction from oxides in high-quality manganese concentrate, choosing reducing agents that can significantly enhance manganese extraction, and developing effective technologies for preparing and using high-quality manganese concentrate.
Materials and methods
We determined the phase and chemical compositions of high-quality manganese concentrate using chemical and X-ray phase analysis methods.
The research [1] showed that the metallothermic reduction of manganese from oxides is significantly accelerated with the presence of marokite (Ca, Mg)Mn2O4 and calcium and magnesium manganites (Ca, Mg)MnO3 , which can be obtained from the high-quality manganese concentrate. A constant amount of marokite and calcium and magnesium manganites is required in the initial manganese-containing material for stable reduction of manganese. Marokite-manganite concentrate and monophase synthetic manganese material (CaMnO3 ) can be obtained using a technology that includes the mechanical and thermal treatment of a mixture of high-quality manganese concentrate and calcined dolomite or lime.
Marokite-manganite concentrate can be used for alloying steel with manganese when it is smelted in an electric furnace or ladle furnace unit, while monophase synthetic manganese material is efficient for the production of metallic manganese.
For thermodynamic modeling of manganese reduction from oxides of high-quality manganese concentrate and marokite-manganite concentrate, we used the Terra software package. This software, based on the maximum entropy principle, finds the equilibrium composition of a multicomponent, heterogeneous thermodynamic system under high-temperature conditions [20].
To determine the technological parameters of the mixtures for steel treatment in an electrical steel-melting furnace, briquettes were fabricated in the ladle furnace unit from marokite-manganite concentrate and powder of spontaneously scattered alloy FS45Mn25 (25 % Mn and 45 % Si) [1]. The binder consisted of 23.2 % ashes from combined heat and power plants (8.88 % Al2O3 ; 23.98 % SiO2 ; 0.56 % TiO2 ; 45.85 % CaO; 4.98 % MgO; 6.32 % FeO; 8.18 % Fe2O3 ; 1.82 % losses on ignition and water (the rest).
The briquettes were melted in a Tamman furnace at a temperature of 1773 – 1823 K. After holding for 5 min, the metal and slag were drained and analyzed.
Using monophase synthetic material CaMnO3 to obtain metallic manganese through aluminothermic treatment enhances the process’s thermality (since manganese in this compound is in a higher oxidation state), allows smelting to be conducted using an out-of-furnace process, and increases manganese recovery.
A software application was developed to calculate the charge composition, smelting products, and the specific thermal effect of the aluminothermic treatment of metallic manganese, based on the stoichiometric equations of heat balance in the metallothermic process.
For experimental melting, the charge included high-quality concentrate, monophase material (CaMnO3 ), and aluminum powder. The melting was carried out in a crucible with an upper opening.
Results and discussion
The averaged chemical composition of the high-quality manganese concentrate is as follows: 59.50 % Mntot ; 0.28 % Fetot ; 5.35 % CaO; 4.00 % CaCl2; less than 1.00 % SiO; and less than 0.01 % P. The results of X-ray phase analysis showed that manganese in the high-quality concentrate mainly exists in the form of Mn3O4 , and it also contains small quantities of α-manganese, manganosite MnO, and calcium chloride CaCl2 .
The study of the carbonothermal reduction of manganese in the Mn3О4 – C system, in the absence of iron, showed that the manganese reduction begins at temperatures above 1723 K with carbon consumption exceeding 1.5 moles. At this temperature, manganese starts to evaporate. At 1723 K, with excess carbon, manganese carbide (Mn7C3 ) is present in the system but disappears with increasing temperature. Complete reduction of manganese occurs at a carbon consumption of 2 moles.
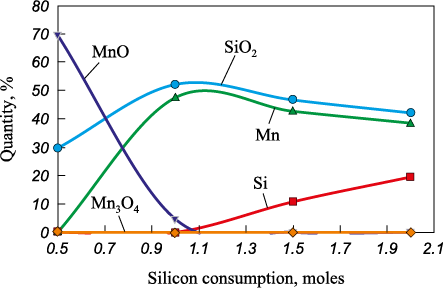
Calculations in the Mn3O4 – Si system showed that manganese can be reduced by silicon over the entire specified temperature range. The research results, presented in Fig. 1, indicate that complete reduction of manganese occurs at a silicon molar rate of 1 mole. This value corresponds to the maximum (47 %) manganese content in the system, which decreases with increasing consumption of the reducing agent due to dilution with excess silicon.
Fig. 1. Dependence of equilibrium compositions |
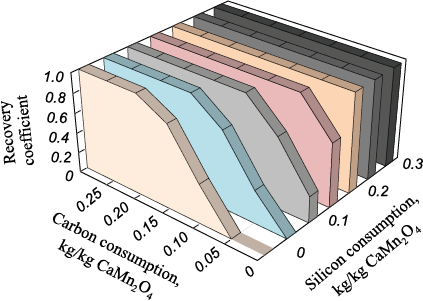
During thermodynamic modeling of the manganese reduction from marokite oxides, calculations were performed for 1 kg of CaMn2O4 , with the amount of reducing agents (carbon and silicon) ranging from 0 to 0.30 kg/kg marokite at temperatures from 1273 to 2273 K.
The results demonstrated that when carbon is used as a reducing agent, reduction begins at a temperature of 1623 K with carbon consumption exceeding 0.05 kg/kg marokite, and ends at 1723 K. When manganese is reduced from marokite oxides with silicon, the process is independent of temperature within the given range, meaning that at steelmaking temperatures, manganese reduction depends solely on the consumption of the reducing agent.
During the joint reduction of manganese from marokite with carbon and silicon at steelmaking temperatures, manganese is found in the form of metallic manganese in both condensed and gaseous phases, with no manganese carbide present.
Fig. 2 shows the dependence of the manganese recovery coefficient on the consumption of carbon and silicon at T = 1923 K, proving that when the consumption of reducing agents exceeds 0.2 kg/kg marokite, manganese is completely reduced.
Fig. 2. Manganese recovery coefficient at combined reduction |
Thus, any of the considered reducing agents or their combinations in specific proportions can be utilized as a reducing agent when manganese-containing oxide materials are used for steel treatment.
The results of the phase analysis of marokite-manganite concentrate samples obtained by heat treatment of mixtures of the high-quality manganese concentrate and calcined flux metal, and their weighing after two weeks of storage in air are presented in Table 1.
Table 1. Results of phase analysis of the samples
| ||||||||||||||||||||||||||||||||||
The mixture of high-quality manganese concentrate and calcined dolomite, after being held for 2 h at a temperature of 1223 K, transforms into marokite-manganite concentrate, which is practically non-hygroscopic in air as it does not contain free calcium oxides.
Experimental data on the manganese reduction from briquettes with the marokite-manganite concentrate are presented in Table 2. When the marokite-manganite concentrate is obtained from the high-quality manganese concentrate and dolomite, steel treatment with manganese-containing materials becomes practically waste-free.
Table 2. Average results of experiments on reduction of briquettes
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The ratio (CaO + 1.4MgO)/Mn in the marokite-manganite concentrate can range from 0.5 to 1.0. However, during smelting, the best results are obtained with a ratio of (CaO + 1.4MgO)/Mn in the marokite-manganite concentrate between 0.50 and 0.72. This is due to the lower consumption of the reducing agent (silicon), which in turn reduces the slag ratio. At (CaO + 1.4MgO)/Mn = 0.51 to 1.00, all manganese is bound into calcium and magnesium manganites and marokite, ensuring its complete reduction. At (CaO + 1.4MgO)/Mn < 0.50, free manganese oxides appear, leading to increased manganese loss during reduction. Conversely, when this ratio exceeds 0.72, silicon consumption for reduction and the slag ratio increase, though the reduction value remains high if the ratio is less than 1.0.
Based on the results of thermodynamic calculations and experimental studies, the following technological parameters for steel treatment with marokite-manganite concentrate in an electric furnace and a ladle-furnace unit are proposed: the concentrate should be applied to the surface of the metal, and initially, the metal and slag should be thoroughly deoxidized to reduce the total oxidation of the metal-slag systems. The actual reducing agent that reduces manganese from the melt of the marokite-manganite concentrate is silicon. To conserve silicon, manganese should be initially reduced with carbon introduced with coke onto the surface of the manganese-containing oxide melt.
When using high-quality manganese concentrate to produce metallic manganese through thermochemical synthesis, a monophasic material is obtained, and the results of its X-ray phase analysis are presented in Table 3.
Table 3. Results of X-ray phase analysis of synthesized monophase material
|
The manganese reduction by aluminum from the synthesized material is accompanied by a significant release of heat and can be represented by the equation
CaMnO3 + Al → [CaO·Al2O3 + 12CaO·7Al2O3] + Mn.
The resulting Al2O3 reacts with CaO to form low-melting aluminates. Therefore, during reduction, manganese losses can theoretically be minimal.
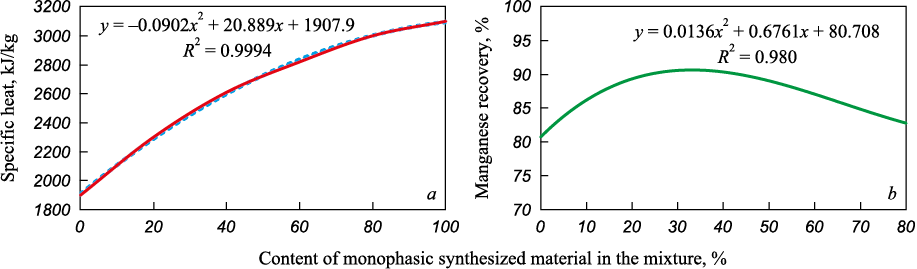
Since a mixture of high-quality manganese concentrate and monophase synthetic material CaMnO3 was used as the initial manganese-containing material, the optimal ratio of the mixture components had to be determined. Heat balances of the aluminothermic melting of metallic manganese were calculated using the developed method. The calculation results are presented in Fig. 3, a.
Fig. 3. Зависимость удельной теплоты процесса (а) и выхода марганца (б) |
To achieve a specific thermal effect of 2500 – 2600 kJ/kg of charge required for the spontaneous process and good separation of metal and slag, the charge should contain 25 – 35 % of the synthesized monophase material CaMnO3 and 65 – 75 % of the high-quality manganese concentrate, as confirmed by experimental data (Fig. 3, b).
As a result of experimental melting, the metal was obtained with the chemical composition presented in Table 4. The results obtained clearly show that the alloy’s chemical composition meets the requirements of GOST 6008 – 80.
Table 4. Chemical composition of the experimental metal
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
It should be noted that metallic manganese has a low content of harmful impurities (phosphorus and sulfur), and the iron content does not exceed 1 %. The manganese recovery from the concentrate averaged approximately 90 %, and the slag ratio ranged from 2.30 to 2.65.
During the experiments, the melt temperature was about 2300 – 2373 K, with the optimal ratio of high-quality concentrate to monophase material (CaMnO3 ) being 6.5 – 7.5 to 3.5 – 2.5.
The recovery of manganese during smelting from the high-quality manganese concentrate reached 85.3 – 89.3 %, which significantly exceeds the manganese recovery when producing metallic manganese from peroxide manganese ores by aluminum thermal out-of-furnace treatment (69 – 72 %). The beneficial use of aluminum was 94 – 96 %.
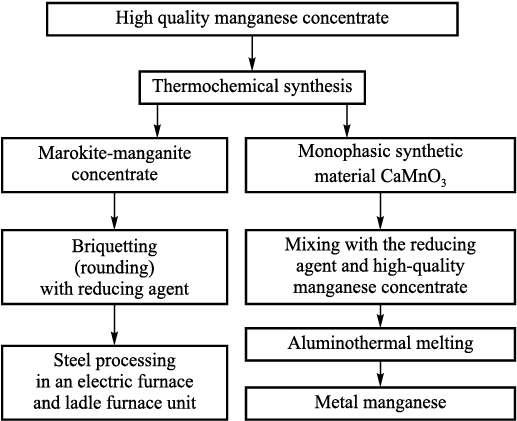
Based on the thermodynamic and experimental studies, we developed the process diagram (Fig. 4) for using high-quality manganese concentrate obtained through hydrometallurgical enrichment of ferromanganese ores from Kuzbass.
Fig. 4. Process diagram of application of high-quality manganese concentrate |
Conclusions
The results of experimental studies enabled development of a technology for producing marokite-manganite concentrate and single-phase synthetic material (CaMnO3 ), the use of which enhances manganese recovery to 90 ‒ 92 %.
Thermodynamic modeling was used to determine the optimal temperatures and consumption of reducing agents (carbon and silicon) to ensure manganese reduction from the oxides of high-quality manganese concentrate obtained from the hydrometallurgical enrichment of ferromanganese ores, as well as from marokite-manganite concentrate. We established that any of the considered reducing agents or their combinations in specific proportions can be used as a reducing agent when oxide manganese-containing materials are used for steel treatment.
Based on the results of thermodynamic calculations and experimental studies, we proposed technological parameters for processing steel with marokite-manganite concentrate in an electric furnace and a ladle furnace unit. To obtain metallic manganese using the out-of-furnace aluminum thermal treatment, optimal processing methods were developed involving the monophasic synthetic material (CaMnO3 ) and high-quality manganese concentrate, which will increase manganese recovery to 90 %.
References
1. Rozhikhina I.D., Nokhrina O.I. Production of Manganese-Containing Materials and Alloys Using Ores from Deposits in Western Siberia. Novokuznetsk: RC SibSIU; 2007:172. (In Russ.).
2. Nokhrina O.I., Rozhikhina I.D., Edil’baev A.I., Edil’baev B.A. Manganese ores of the Kemerovo region – Kuzbass and methods of their enrichment. Izvestiya. Ferrous Metallurgy. 2020;63(5):344–350. (In Russ.). https://doi.org/10.17073/0368-0797-2020-5-344-350
3. Tigunov L.P., Smirnov L.A., Menadisieva R.A. Manganese: Geology, Production, Application. Ekaterinburg: NSA; 2006:183. (In Russ.).
4. Nokhrina O.I., Rozhikhina I.D., Golodova M.A., Izrail’skii A.O. Study of Kuzbass iron-manganese ores enrichment possibilities. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2020;76(9):904–909. (In Russ.).
5. Sutyrin Yu.E. Analysis of the state of hydrometallurgical processing of manganese raw materials. Natsional’naya metallurgiya. 2003;(2):99–104. (In Russ.).
6. Chernobrovin V.P., Mizin V.G., Sirina T.P., Dashevskii V.Ya. Complex Processing of Carbonate Manganese Raw Materials: Chemistry and Technology. Chelyabinsk: SUSU; 2009:294. (In Russ.).
7. Tigunov L.P., Ozhogina E.G., Litvintsev E.G., Bronitskaya E.S., Anufrieva S.I., Kalish E.A. Up-to-date technologies for concentration and hydrometallurgical processing of manganese ores. Gornyi zhurnal. 2007;(2):78–84. (In Russ.).
8. Kurkov A.V., Mamoshin M.Yu., Rogozhin A.A. Breakthrough Hydrometallurgical Processes for the Sustainable Development of Mineral Processing Technologies. Moscow: VIMS; 2019:106. (In Russ.).
9. Dreisinger D. Keynote address: Hydrometallurgical process development for complex ores and concentrates. Journal of the Southern African Institute of Mining and Metallurgy. 2009;109(5):253–271.
10. Hatk P.K., Sukla L.B., Das S.C. Aqueous SO2 leaching studies on Nishikhal manganese ore through factorial experiment. Hydrometallurgy. 2000;54(2–3):217–228.
11. Trifoni M., Toso L., Vegliu F. Reductive leaching of manganiferous ores by glucose and H2SO4 : effect of alcohols. Hydrometallurgy. 2001;59(1):1–14. https://doi.org/10.1016/S0304-386X(00)00138-9
12. Ding P., Liu Q., Pang W. A review of manganese ore beneficiation: Situation and development. Applied Mechanics and Materials. 2013;380–384:4431–4433. http://dx.doi.org/10.4028/www.scientific.net/AMM.380-384.4431
13. Yang Z.Z., Li G.Q., Huang C.G., Ding J. Mn ore smelting reduction based on double slag operation in BOF. Applied Mechanics and Materials. 2013;753-755:76–80. http://dx.doi.org/10.4028/www.scientific.net/AMR.753-755.76
14. Pan M.C., Liu X.L., Zou R., Huang J., Han J.C. Study of heat treatment technology on medium-carbon-low-alloy-steel large hammer formation of gradient performance. Advanced Materials Research. 2014;881-883:1288–1292. http://dx.doi.org/10.4028/www.scientific.net/AMR.881-883.1288
15. Ayala J., Fernandez B. Recovery of manganese from silicomanganese slag by means of a hydrometallurgical process. Hydrometallurgy. 2015;158:68–73. https://doi.org/10.1016/j.hydromet.2015.10.007
16. Vegliо F., Trifoni M., Abbruzzese C., Toro L. Column leaching of a manganese dioxide ore: a study by using fractional factorial design. Hydrometallurgy. 2001:59(1):31–44. http://dx.doi.org/10.1016/S0304-386X(00)00139-0
17. Kang T.T., Liu Y., Huang Y.B., Dong J., Huang Q., Li Y. Synthesis and dephosphorization of iron manganese composite oxide by acid leaching on iron manganese ore. Advanced Materials Research. 2012;554–556:489–493. http://dx.doi.org/10.4028/www.scientific.net/AMR.554-556.489
18. Sun D., Li M.L., Li C.H., Cul R., Zheng X.Y. A green enriching process of Mn from low grade ore of manganese carbonate. Applied Mechanics and Materials. 2014;644–650: 5427–5430. http://dx.doi.org/10.4028/www.scientific.net/AMM.644-650.5427
19. Nokhrina O.I., Rozhikhina I.D., Golodova M.A. Production of high-quality concentrates by method of hydrometallurgical concentration of manganese ores. Russian Internet Journal of Industrial Engineering. 2023;10(1):47–51. (In Russ.).
20. Trusov B.G. TERRA software system for modeling phase and chemical equilibria at high temperatures. In: III Int. Symp.”Combustion and Plasma Chemistry”. August 24-26, 2005, Almaty, Kazakhstan. Almaty: Kazak universiteti; 2005:52–57. (In Russ.).
About the Authors
I. A. RybenkoRussian Federation
Inna A. Rybenko, Dr. Sci. (Eng.), Prof., Head of the Chair of Applied Information Technologies and Programming
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
I. D. Rozhikhina
Russian Federation
Irina D. Rozhikhina, Dr. Sci. (Eng.), Prof. of the Chair of Ferrous Metallurgy
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
O. I. Nokhrina
Russian Federation
Ol’ga I. Nokhrina, Dr. Sci. (Eng.), Prof. of the Chair of Ferrous Metallurgy
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
M. A. Golodova
Russian Federation
Marina A. Golodova, Cand. Sci. (Eng.), Assist. Prof. of the Chair of Architecture
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
Review
For citations:
Rybenko I.A., Rozhikhina I.D., Nokhrina O.I., Golodova M.A. Rational application of high quality manganese concentrate. Izvestiya. Ferrous Metallurgy. 2024;67(2):237-244. https://doi.org/10.17073/0368-0797-2024-2-237-244