Scroll to:
Joint processing of perovskite and ilmenite concentrates. Part 1. Chemical-mineralogical (material) characteristics of perovskite and ilmenite concentrates
https://doi.org/10.17073/0368-0797-2024-1-27-36
Abstract
Russia has an impressive titanium mineral resource while the contribution into the global production of titanium concentrates is quite insignificant. The current annual demand of Russian enterprises for titanium raw materials is 40 times higher than its production. To improve and launch the processing of domestic titanium raw materials characterized by low quality and complex polymineral composition, new process solutions are required. These solutions should aim at the full extraction of TiO2 and related valuable components from the ore deposits whose development is planned or already started (for example, Afrikanda – perovskite-titanomagnetite deposit located on the Kola Peninsula). This report presents the results of studying the chemical and mineral compositions of perovskite and ilmenite concentrates with the purpose to assess the possibility of their joint processing using carbothermic reduction melting. Emission spectrometry, X-ray diffraction, electron microscopy, and X-ray spectral microanalysis were applied in these studies. It was found that the basis of the ilmenite gravity concentrate sample is modified ilmenite represented by leucoxenization products – pseudorutile and rutile, with their total content in the concentrate to be about 80 wt. %. Composition of other minerals (alumochromite, chromite, magnetite) includes titanium in the form of impurities – 2 – 3 wt. %. In the perovskite flotation concentrate sample titanium is contained in perovskite and titanite making up the bulk of the ore minerals of the concentrate. As for rare and rare-earth elements contained in the ilmenite sample – monazite having up to 33 wt. % Ce, and zircon were found. Perovskite sample contains rare-earth elements (REE concentration in wt. %) in loparite-(Ce) (22.8), aluminocerite-(Ce) (46.2), anсylite-(Ce) (51.3), torite (22.3), as well as in the main mineral – perovskite (2.8). With the exception of perovskite and loparite-(Ce), other REE-containing minerals are rare, and their share in total does not exceed 1 wt. %
Keywords
For citations:
Fedorov S.A., Udoeva L.Yu., Vusikhis A.S., Pikulin K.V., Cherepanova L.A. Joint processing of perovskite and ilmenite concentrates. Part 1. Chemical-mineralogical (material) characteristics of perovskite and ilmenite concentrates. Izvestiya. Ferrous Metallurgy. 2024;67(1):27-36. https://doi.org/10.17073/0368-0797-2024-1-27-36
Introduction
Russia possesses a significant titanium mineral resource [1; 2]. According to 2019 data from the Ministry of Natural Resources and Environment, Russia accounts for 12.5 % of the world’s reserves. However, its contribution to the global production of titanium concentrates is relatively minor – only 0.04 % (about 9000 tons). Tugansky MPP “Ilmenite,” the sole producer of titanium concentrate in Russia, manages this production. Concurrently, data from FSBI VIMS indicates that the current annual demand for titanium raw materials by Russian enterprises is approximately 365,000 tons. Nearly all of this demand is met by imported ilmenite (about 340,000 tons) and rutile (about 12,000 tons) concentrates. Only 13,000 tons of domestic raw material, the loparite concentrate, are supplied to Russian companies.
The primary reason for this situation is that the main reserves of titanium in the Russian Federation, estimated at 587.6 million tons of TiO2 as of January 2022, are found in polymetallic ores. The efficiency of processing these ores is determined by the potential for associated extraction of other valuable components. One of the unique Russian deposits of complex polymetallic ores is located on the Kola Peninsula, in the Afrikanda settlement. These ores are perovskite-titanomagnetite, containing not only titanium and iron but also rare elements (tantalum, niobium), rare-earth elements (lanthanum, cerium, etc.), and radioactive metals (thorium), with total reserves exceeding 626 million tons. The content of perovskite is 21.5 %, and titanomagnetite is 2.5 % [4 – 6]. The Afrikanda deposit was discovered a century ago, in 1917. In the 1930s, there was an attempt to obtain concentrates for titanium and thorium production, and in the 1950s, for the needs of ferrous metallurgy. Both projects failed, and in 1972, the titanium ore reserves of Afrikanda were removed from the State Register of Reserves.
The current economic and social level of the Kola Region allows for the reconsideration of the cost-effective development of the Afrikanda deposit. The Kola Scientific Center of the Russian Academy of Sciences has developed an effective magnetic-flotation scheme for enriching perovskite-titanomagnetite ores. This scheme includes magnetic separation of the initial ore to extract titanomagnetite concentrate and flotation of the non-magnetic fraction to extract perovskite concentrate [5; 6]. The titanomagnetite concentrate, containing up to 8 % TiO2 , is primarily of interest as raw material for ferrous metallurgy, suitable for processing in a classic manner, including blast furnace smelting, as well as using the electro-thermal method with the potential for associated extraction of vanadium [8 – 10].
Perovskite concentrates represent unconventional titanium raw materials that necessitate complex processing to produce titanium dioxide and compounds of associated components. For Afrikanda concentrates, several hydrometallurgical technologies have been developed. These technologies involve decomposing the concentrates with mineral acids, converting all components into salt solutions or hydrate products, and then extracting titanium dioxide, rare metals, and rare-earth elements [11; 12]. The proposed schemes, which have been tested on a pilot scale, are realistic and hold promise. However, like all hydrometallurgical technologies, they entail lengthy multi-stage processes, including leaching, precipitation, thickening, filtration, etc., and lead to the accumulation of hazardous effluents needing disposal. Researchers [13] have explored pyrometallurgical solutions to the processing of perovskite concentrates, attempting to address the challenges associated with hydrometallurgical methods. One proposal involves obtaining titanium carbide and metallic calcium through a two-stage reduction smelting process. It is important to note that perovskite raw materials are not utilized for titanium production outside of Russia.
Ilmenite ores, which are significantly found in the Gremyakha-Vyrmes Massif on the Kola Peninsula, differ from perovskite ores in that they satisfy approximately 90 % of the global demand for titanium-containing raw materials used in the production of metallic titanium, titanium dioxide, and carbide. To process the relatively resistant ilmenite mineral, various methods are employed, including pyrometallurgical processes, acid decomposition at high temperatures, and combined approaches [14 – 16]. Most pyrometallurgical technologies rely on reduction smelting using carbon-containing [17 – 19] or combined [20] reducing agents, which is enhanced by the pre-oxidation of the ilmenite concentrate [21; 22]. It is observed [23] that electric smelting of ilmenite concentrates with coal results in the formation of slags with a titanium content similar to that of perovskite but are more readily dissolved by acids. To lower the reduction smelting temperature of ilmenite in the ore-thermal furnace, calcium oxide is added to the mixture. This addition helps to adjust the ratio of TiO2 and CaTiO3 , ensuring a slag melting temperature of 1400 – 1450 °C [24]. It is proposed that achieving the desired TiO2/CaTiO3 in the titanium slag could be more efficiently accomplished by incorporating perovskite concentrate, based on calcium titanate CaTiO3 , rather than calcium oxide, into the ilmenite concentrate smelting charge. This approach will not significantly alter the titanium content in the slag but will facilitate the processing of titanium raw materials with diverse mineral compositions of the concentrate ore components within a unified workflow system.
In our study, we explored the feasibility of jointly processing perovskite and ilmenite concentrates through carbothermic reduction smelting. This method aims to extract rare metals into cast iron and generate a titanium-rich slag that is amenable to the hydrometallurgical extraction of titanium and rare earth elements. Given that the phase composition and distribution of components within the mineral components of titanium-containing concentrates play a crucial role in dictating the interaction mechanisms during processing, the initial phase of our research concentrated on determining the chemical and material compositions. We also examined the microstructure of perovskite and ilmenite concentrate samples selected for analysis.
Materials and methods
To conduct chemical analysis of the averaged concentrate samples, a Spectroflame Modula S inductively coupled plasma atomic emission spectrometer (ICP-AES) was utilized.
The phase composition of the samples was determined using X-ray powder diffraction (XRD) with a Shimadzu XRD 7000C diffractometer. The diffractometer operated under the following conditions: CuKα radiation (λ = 0.154051 nm), with a voltage of 34 kV and a current of 40 mA. Data were collected across a 2θ range from 20 to 80 – 90°, in 0.02° increments, with a point exposure time of 2.0 s. Phase identification was performed using the International Centre for Diffraction Data (ICDD) PDF–4 database [25]. The quantitative assessment of the phase composition was carried out through Rietveld full-profile analysis [26], employing the TOPAS software [27].
For the investigation of the microstructure and the elemental analysis of minerals composing the concentrates, a Carl Zeiss EVO 40 scanning electron microscope (SEM) equipped with an HKL Channel 5 EBSD (Premium) energy dispersive attachment was used. The images of the samples’ microstructure were captured using the backscattered electrons (BSE) detector.
Results and discussion
The ilmenite sample closely mirrors the major elements found in the gravity concentrate from the Gremyakha-Vyrmes deposit [28], while the perovskite material corresponds to a rough flotation concentrate from the Afrikanda deposit [29]. The chemical analysis results of the concentrate samples are presented in Table 1.
Table 1. Chemical composition of the ilmenite and perovskite concentrates
| |||||||||||||||||||||||||||||||||||||||
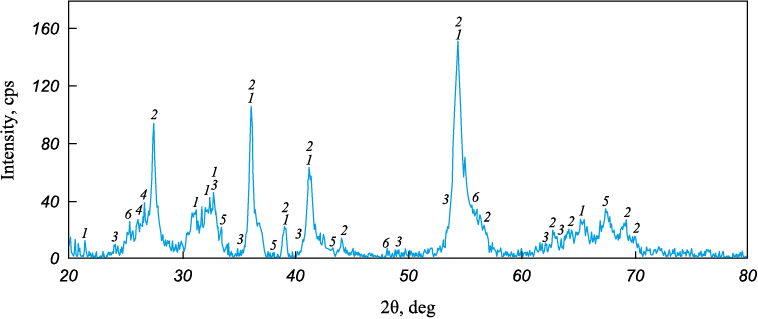
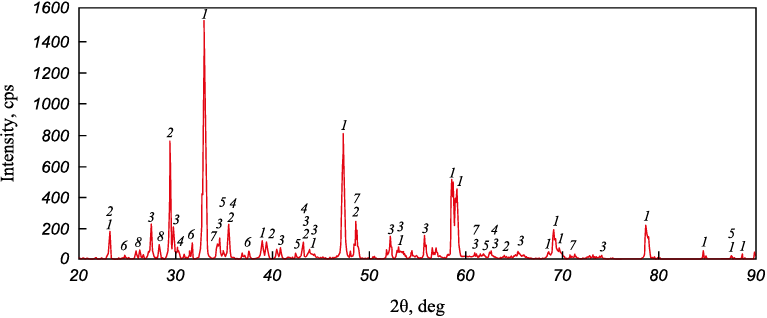
According to the X-ray phase study (Fig. 1, Table 2), the ilmenite concentrate primarily consists of Fe2Ti3O9 pseudorutile (48 wt. %) and rutile (29 wt. %). This composition is typical for concentrates of the so-called altered ilmenite, which forms as a result of its leucoxenization. The actual content of ilmenite in the concentrate is only 7 wt. %. Aluminum and silicon are concentrated in staurolite and sillimanite. The main mineral constituents of the perovskite concentrate (Fig. 2, Table 3) are perovskite (56 %), calcite (13 %), and titanite (11 %).
Fig. 1. XRD pattern of the ilmenite concentrate:
Table 2. Phase composition of the ilmenite concentrate
Fig. 2. XRD pattern of the perovskite concentrate:
Table 3. Phase composition of the perovskite concentrate
|
Iron is present in the forms of magnetite, ulvospinel, and fayalite, while silicon is found in fayalite and quartz. Overall, the mineral composition of the investigated materials aligns with their chemical composition.
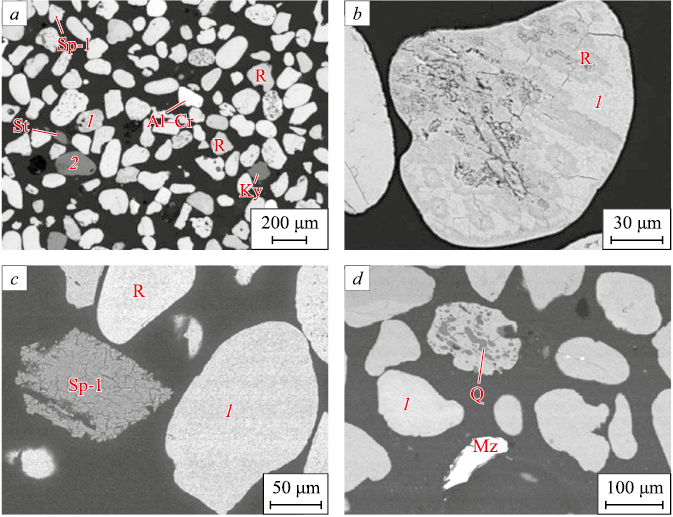
The ilmenite concentrate sample is a loose, fine-grained material obtained during the gravity concentration of the original ore. The majority of the grains exhibit a well-pelletized shape, with their sizes ranging from 10 to 300 μm. For most grains, this parameter lies between 150 and 200 μm (Fig. 3). The concentrate is primarily composed of pseudorutile, rutile, staurolite, and quartz. The study also revealed the presence of minerals from the spinel group (including picotite, alumochromite, chromite, hercynite, aluminomagnetite, gahnite, and magnetite), monazite, Mg and Fe aluminosilicates, sillimanite, and zircon. This complex assortment of ore components is characteristic of ilmenite placers [30].
Fig. 3. BSE-images of the ilmenite concentrate: |
Pseudorutile is characterized by well-rounded grains (Fig. 3, a), which are often ellipsoidal and spherical in shape, according to the A.V. Khabakov scale [31]. Quartz inclusions, as well as less frequently zircon and magnetite inclusions, occupy the recesses on the surfaces of pseudorutile grains and fill voids of irregular or prismatic shapes. Some grains exhibit a large number of unfilled pores, indicating a porous texture. The chemical composition of the mineral varies, with magnesium admixtures reaching up to 1.2 wt. %, and manganese up to 2.6 wt. %.
Rutile, another major titanium mineral in the concentrate, appears in elongated, prismatic, and isometric shapes, ranging from well- to medium-rounded grains (Fig. 3, a). These grains are comparable in size to pseudorutile grains, measuring 100 – 200 µm. Rutile contains iron impurities, with a maximum content of 9.6 wt. % and an average of 2.2 wt. %. Some medium-rounded grains, which have a cross-sectional shape close to rhombic, are likely to be anatase, another polymorphic modification of TiO2 . It is noteworthy that polymineral grains with clear signs of secondary alteration of ilmenite, known as leucoxenization, are present. These grains are composed of pseudorutile and rutile and often include quartz inclusions (Fig. 3, b). To identify the products of ilmenite leucoxenization, we utilized the criterion proposed in [32], specifically the ratio of Ti/(Ti + Fe), which averaged 0.68 for pseudorutile grains and 0.96 for rutile in the studied sample of ilmenite concentrate.
In the studied sample of ilmenite concentrate, seven minerals from the spinel group were identified: picotite ((Fe, Mg)(Al, Cr)2O4 ), aluminochromite (Fе(Сr, А1)2O4 ), chromite (FeCr2O4 ), ganite (ZnAl2O4 ), hercynite (FeAl2O4), aluminomagnetite (Fe2\(^+\)(Fe3\(^+\), Al)2O4 ) and magnetite (Fe2\(^+\)Fe\(_2^{3+}\)O4). The grains of these minerals ranging in size from 50 to 200 µm, are poorly rounded and have an isometric shape. Octahedral crystals and their fragments also occur (Fig. 3, a). The chemical composition of minerals varies within the plane of section. Picotite, most commonly found in association with rutile and pseudorutile (Fig. 3, c), shows variability in its composition. Alumochromite and chromite, with titanium admixtures of 0.2 – 3.0 wt. % and 2.6 wt. % respectively, are encountered less frequently. Ganite and magnetite, both with a titanium admixture of 2.6 wt. %, along with spinel exhibiting a zonal structure, are observed as singular grains. The core of the spinel is composed of hercinite, while the periphery consists of aluminomagnetite.
Among the accessory and other ore minerals, monazite (CePO4) and zircon (Zr[SiO4]) were identified. Monazite is represented by elongated wedge-shaped grains, 150 – 200 μm in length, associated with pseudorutile (Fig. 4, d). It contains cerium (27.5 – 47.3 wt. %) and impurities such as lanthanum (up to 13.7 wt. %), neodymium (up to 12.5 wt. %), thorium (up to 7.1 wt. %), and praseodymium (up to 4.3 wt. %). Zircon, found as inclusions in pseudorutile, exhibits a shape close to a tetragonal prism, with grain sizes ranging from 1 to 10 μm in length and up to 5 μm in cross section.
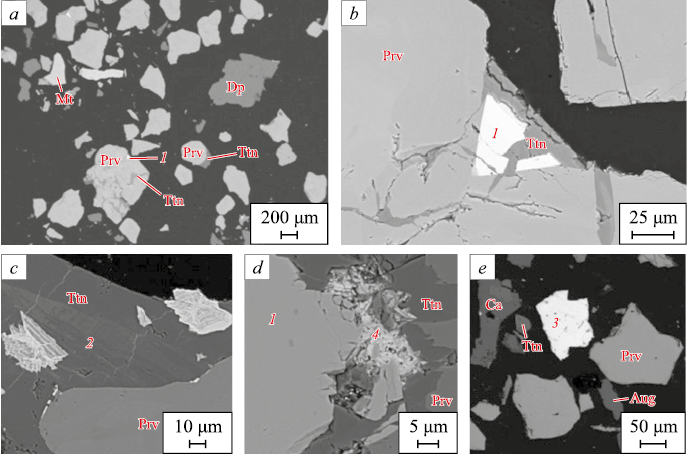
Fig. 4. BSE-images of the perovskite concentrate: |
The concentrate also contains rock-forming minerals such as Mg and Fe aluminosilicate, sillimanite, staurolite, and quartz. Aluminosilicate is present in both isometric and elongated grains, nearing prismatic in shape, with grain sizes ranging from 150 to 300 µm. Sillimanite and staurolite form grains that are poorly to medium rounded and prismatic in shape, with their sizes not exceeding 100 – 200 µm and 200 – 300 µm, respectively. Quartz (Q) occurs exclusively as inclusions in pseudorutile, with sizes ranging from less than 1 to 60 µm (Fig. 3, d).
The perovskite concentrate, a rough flotation concentrate from the Afrikanda deposit, is a loose, crushed material with the grain size ranging from 20 to 300 µm (Fig. 4, a). The grains, predominantly isometric, irregular, and prismatic, are prevalent. The concentrate contains two major titanium minerals, perovskite and titanite, along with loparite-(Ce), aluminocerite-(Ce), ancylite-(Ce), thorite, magnetite, diopside, calcite, ulvospinel, fayalite, phlogopite, enstatite, aegirine, and augite. Perovskite is the most abundant mineral in the concentrate, forming isometric grains that range in size from 20 to 1000 µm (Fig. 4, b), and its shape is sometimes close to cubic. The cracks in perovskite are mostly filled with titanite and rare earth elements, and these cracks can exceed 100 µm.
Titanite is the second most abundant titanium mineral in the sample. It often fills cracks and cavities in perovskite (Fig. 4), but individual wedge-shaped crystals are also found, with sizes ranging from a few micrometers to 200 μm. The mineral contains impurities of iron (0.7 – 5.0 wt. %) and aluminum (0.3 – 2.5 wt. %). Another titanium-bearing mineral, loparite-(Ce), with the general formula (Ce, Na, Ca)(Ti, Nb)O3 , forms crystals that are close in shape to octahedrons and cubes, with sizes ranging from 50 to 120 μm (Fig. 4, b). These crystals occur in very small quantities as impregnations and intergrowths with perovskite and titanite, and less frequently as intergrowths with magnetite. The impregnations can be up to 100 μm in size. Loparite-(Ce) contains cerium (15.4 – 20.5 wt. %), neodymium impurities (3.5 – 6.8 wt. %), thorium (1.2 – 1.6 wt. %), and occasional grains of niobium (3.1 – 8.2 wt. %).
In addition to loparite-(Ce), the perovskite concentrate sample contains three other rare earth element (REE)-bearing minerals in the form of single grains: aluminocerite-(Ce), ancylite-(Ce), and thorite, as indicated by elemental analysis. Aluminocerite-(Ce), with the formula (Ce, Ca)9Al[SiO4]3[SiO3(OH)]4(OH)3 , forms isometrically shaped grains ranging in size from 5 to 100 μm (Fig. 4, c). At high magnification, some of these grains exhibit a layered texture, composed of oriented tabular crystals, zoning, and a structure reminiscent of solid solution decomposition. Ancylite-(Ce) is sometimes found in the central part of the grain. The major impurities in aluminocerite-(Ce) include lanthanum (6.8 – 14.1 wt. %), neodymium (5.8 – 9.8 wt. %), and thorium (1.3 – 3.7 wt. %), with cerium content ranging from 24.3 to 42.1 wt. %.
Ancylite-(Ce), with the formula CeSr(CO3)2(OH)·H2O s characterized by isometric grains and irregularly shaped particles of varying sizes, from less than 2 to 150 μm (Fig. 4, e). It forms inclusions in titanite and perovskite, as well as individual large grains and crystalline aggregates. The cerium content in this mineral ranges from 25.3 to 30.1 wt. %, with impurities including lanthanum (14.4 – 18.3 wt. %) and neodymium (5.7 – 10.4 wt. %). Thorite is represented by prismatic, zonal crystals and their aggregates, with lengths up to 5 μm and thicknesses up to 2 μm (Fig. 4, d). This mineral fills voids and cracks in titanite and perovskite, containing impurities such as yttrium (5.2 – 7.8 wt. %), gadolinium (2.8 – 3.5 wt. %), phosphorus (0.8 – 1.2 wt. %), and aluminum (0.4 – 0.6 wt. %). However, some of the rare earth elements in its chemical composition may be attributable to the surrounding perovskite and loparite.
The last of the ore minerals in the perovskite concentrate is magnetite, which occurs as isomeric grains and fragments of octahedral crystals. The surface of the magnetite displays signs of dissolution, and the mineral contains small admixtures of titanium (0.5 – 0.7 wt. %). In some grains, the lamellar structure resulting from the decomposition of the titanium-magnetite solid solution was observed, with the formation of ulvospinel veinlets.
The rock-forming minerals in the perovskite concentrate sample include calcite, fayalite with a small admixture of magnesium (Fe, Mg)2[SiO4], phlogopite KMg3AlSi3O10(OH)2 , enstatite Mg2[Si2O6 ], aegirine NaFe[Si2O6 ] and augite (Ca, Mg, Fe)2[(Si, Al)2O6 ]. Calcite is frequently found in the sample, forming large rhombohedral crystals and their aggregates (more than 1 mm). The other minerals are present in very small quantities, appearing as prismatic or tabular grains in the form of inclusions or aggregates with titanite or perovskite. Calcite also sometimes forms a crust around magnetite grains and fills cracks in the mineral.
In general, the investigation of the material composition of the perovskite concentrate sample yielded results consistent with data from literary sources [33; 34], except for the minerals containing REE (aluminocerite-(Ce), ancylite-(Ce), and thorite).
Conclusions
The investigations into the chemical and mineralogical composition of ilmenite and perovskite concentrates, along with the assessment of the distribution of their valuable components by structural elements, have enabled us to draw the following conclusions.
Almost all of the titanium in the ilmenite sample is concentrated in pseudorutile and rutile – products of the leucoxenization of ilmenite, i.e., its alteration (weathering), which accounts for a significant portion of the concentrate. Titanium is also found as an impurity in aluminochromite, chromite, and magnetite, with its content not exceeding 2 – 3 wt. %.
The non-metallic part of the ilmenite sample comprises seven minerals from the spinel group, nearly half of which are chromospinelids. Additionally, we found monazite, containing up to 33 wt. % Ce, and zircon; the former occurs as single grains and is found more frequently than zircon.
In the perovskite sample, titanium is primarily represented by the main ore minerals of the concentrate –perovskite and titanite. The rare-earth element cerium is present in the form of specific minerals (loparite-(Ce), aluminocerite-(Ce), ancylite-(Ce), thorite) or as an admixture in perovskite (2.8 wt. % Ce). Except for perovskite and loparite-(Ce), REE-bearing minerals are rare, and their total share in the concentrate does not exceed 1 wt. %. Loparite-(Ce) comprises cerium (18.0 wt. %), neodymium impurities (5.2 wt. %), thorium (1.4 wt. %), and isolated grains of niobium (up to 8.2 wt. %).
References
1. Nikolaev A.I., Larichkin F.D., Gerasimova L.G., etc. Titanium and Its Compounds: Resources, Production, Markets, Prospects. Apatity: Kola Science Centre, RAS; 2011:152. (In Russ.).
2. Bykhovskii L.Z., Tigunov L.P. Titanium raw materials of Russia. Rossiiskii Khimicheskii Zhurnal. 2010;(2(54)): 73–86. (In Russ.).
3. State report on the state and use of mineral resources of the Russian Federation in 2019. Moscow: Ministry of Natural Resources and Ecology of the Russian Federation. 2020:493. (In Russ.).
4. Tochilo M.V., Fedoseev S.V., Larichkin F.D., Novosel’tseva V.D., Gorbovskikh A.V. Prospects and approaches to forming the strategy for the development of the titanium industry in the north-west region of Russia. Sever i Rynok: Formirovanie ekonomicheskogo poryadka. 2019;(3(65)): 99–108. (In Russ.).
5. Andronov G.P., Filimonova N.M., Khokhulya M.S. Separation of titanium-containing minerals by magnetic separation. Vestnik MGTU. 2019;(1(22)):109–119. (In Russ.). https://doi.org/10.21443/1560-9278-2019-22-1-109-119
6. Khokhulya M.S., Gerasimova L.G., Nikolaev A.I. New technological solutions for the preparation and processing of perovskite. In: Trudy Kol’skogo Nauchnogo Tsentra RAN. 2018:196–200. (In Russ.).
7. Leont’ev L.I., Vatolin N.A., Shavrin S.L., Shumakov N.S. Pyrometallurgical Processing of Complex Ores. Moskow: Metallurgiya; 1997:432. (In Russ.).
8. Grishin N.N., Rakitina E.Yu. New elements of technology for processing titanomagnetites of the Kola Peninsula. Trudy Fersmanovskoi Nauchnoi Sessii GI KNTS RAN. 2017;(14):228–231. (In Russ.).
9. Wang S., Chen M., Guo Y., Jiang T., Zhao B. Reduction and smelting of vanadium titanomagnetite metallized pellets. JOM. 2019;71:1144–1149. https://doi.org/10.1007/s11837-018-2863-7
10. Wang S., Guo Y., Chen F., Zheng F., Yang L., Tang M. Behavior of tantalum during the smelting of vanadium titanomagnetite metallized pellets in an electric furnace. JOM. 2019;71:323–328. https://doi.org/10.1007/s11837-018-2932-y
11. Kiselev Yu.G., Shchukina E.S. Solubility of the hydrated product obtained by nitric acid treatment of perovskite in sulfuric acid. Vestnik Kol’skogo Nauchnogo Tsentra RAN. 2017;(2):81–86. (In Russ.).
12. Nikolaev A.I., Gerasimova L.G., Petrov V.B., Maiorov V.G. Perovskite concentrate as a promising non-traditional raw material for production of titanium and rare metal products. Kompleksnoe ispol’zovanie mineral’nogo syr’ya. 2015;(2):26–34. (In Russ.).
13. Budin O.N., Kropachev A.N., Cherepov V.V. Study of technology for producing titanium carbide and calcium metal from perovskite concentrate by carbothermic method. Metallurg. 2020;(4):56–64. (In Russ.).
14. Zhang W., Zhu Z., Chen C.Y. Literature review of titanium metallurgical processes. Hydrometallurgy. 2011;108: 177–188. https://doi.org/10.1016/j.hydromet.2011.04.005
15. Ogasawara T., Veloso de Araujo R.V. Hydrochloric acid leaching of a pre-reduced Brazilian ilmenite concentrate in an autoclave. Hydrometallurgy. 2000;56(2):203–216. https://doi.org/10.1016/S0304-386X(00)00074-8
16. Amer A.M. Alkaline pressure leaching of mechanically activated Rosetta ilmenite concentrate. Hydrometallurgy. 2002;67(2):125–133. https://doi.org/10.1016/S0304-386X(02)00164-0
17. Wang Y.-M., Yuan Z.-F., Guo Z.-C., Tan Q.-Q., Li Z.-Y., Jiang W.-Z. Reduction mechanism of natural ilmenite with graphite. Transactions of Nonferrous Metals Society of China. 2008;18(4):962–968. https://doi.org/10.1016/S1003-6326(08)60166-1
18. Gou H.-P., Zhang G.-H., Hu X.-J., Chou K.-Ch. Kinetic study on carbothermic reduction of ilmenite with activated carbon. Transactions of Nonferrous Metals Society of China. 2017;27(8):1856–1861. https://doi.org/10.1016/S1003-6326(17)60209-7
19. Lv W., Bai C., Lv X., Hu K., Lv X., Xiang J., Song B. Carbothermic reduction of ilmenite concentrate in semi-molten state by adding sodium sulfate. Powder Technology. 2018;340:354–361. https://doi.org/10.1016/j.powtec.2018.09.043
20. Samal S.K., Mishra B., Mishra S.C. Carboaluminothermic production of ferrotitanium from ilmenite through thermal plasma. Journal of Sustainable Metallurgy. 2020;(6): 563–575. https://doi.org/10.1007/s40831-020-00292-5
21. Zhang G., Ostrovski O. Effect of pre-oxidation and sintering on properties of ilmenite concentrates. International Journal of Mineral Processing. 2002;64(4):201–218. https://doi.org/10.1016/S0301-7516(01)00055-2
22. Gou H.P., Zhang G.H., Chou K.C. Influence of pre-oxidation on carbothermic reduction process of ilmenite concentrate. ISIJ International. 2015;55(5):928–933. https://doi.org/10.2355/isijinternational.55.928
23. Reznichenko V.A., Shabalin L.I. Titanomagnetites: Deposits, Metallurgy, Chemical Technology. Moscow: Nauka; 1986:297. (In Russ.).
24. Slag Atlas, 2nd ed. Düsseldorf: Verlag Stahleisen GmbH; 1995:618.
25. Powder Diffraction File PDF4+ ICDD. Release 2016.
26. Rietveld H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallographica. 1967;22(1):151–152. https://doi.org/10.1107/S0365110X67000234
27. DIFFRACPlus: TOPASBruker AXS GmbH. Karlsruhe, Germany; 2008.
28. Reznichenko V.A., Averin V.V., Olyunina T.V. Titanates: Scientific Foundations, Technology, Production. Moscow: Nauka; 2010:267. (In Russ.).
29. Gerasimova L.G., Mel’nik N.A., Nikolaev A.I., Petrov V.B., etc. Hydrochloric acid technology of perovskite concentrate and its radiation assessment. Ekologiya promyshlennogo proizvodstva. 2015;(1(89)):54–58. (In Russ.).
30. Levchenko E.N. Specific features of the mineral composition of titanium-zirconium placers in Russia. Lithology and Mineral Resources. 2006;41(2):117–136. https://doi.org/10.1134/S0024490206020039
31. Kuznetsov A.G., Pashkova N.G. Morphology of large-block material of beaches of the shores of the Tarkhankut peninsula (Crimea). Uchenye zapiski Krymskogo Federal’nogo Universiteta imeni V.I. Vernadskogo. Seriya “Geografiya. Geologiya”. 2015;(1(67)):81–90. (In Russ.).
32. Kotova O.B., Ozhogina E.G., Ponaryadov A.V. Technological mineralogy: development of a comprehensive assessment of titanium ores (exemplified by the Pizhemskoye deposit). Journal of Mining Institute. 2022;256:632–641. (In Russ.). https://doi.org/10.31897/PMI.2022.78
33. Sokolov S.V. Perovskite and titanite – possible unconventional sources of titanium (on the example of the Afrikanda deposit). In: Technological Mineralogy in Assessing the Quality of Mineral Raw Materials of Natural and Man-Made Origin. Coll. of reports of the XIV Russ. Seminar on Technological Mineralogy, Moscow, April 5-6, 2022. Petrozavodsk: Kola Science Centre, RAS; 2022:78–81. (In Russ.).
34. Potter N.J., Ferguson M.R., Kamenetsky V.S., Chakhmouradian A.R., Sharygin V.V., Thompson J.M., Goemann K. Textural evolution of perovskite in the Afrikanda alkaline–ultramafic complex, Kola Peninsula, Russia. Contributions to Mineralogy and Petrology. 2018;173:100. https://doi.org/10.1007/s00410-018-1531-9
About the Authors
S. A. FedorovRussian Federation
Sergei A. Fedorov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Nonferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. Yu. Udoeva
Russian Federation
Lyudmila Yu. Udoeva, Cand. Sci. (Eng.), Leading Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. S. Vusikhis
Russian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
K. V. Pikulin
Russian Federation
Kirill V. Pikulin, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. A. Cherepanova
Russian Federation
Lyubov’ A. Cherepanova, Cand. Sci. (Chem.), Research Associate of the Laboratory of Statics and Kinetics of Processes
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Fedorov S.A., Udoeva L.Yu., Vusikhis A.S., Pikulin K.V., Cherepanova L.A. Joint processing of perovskite and ilmenite concentrates. Part 1. Chemical-mineralogical (material) characteristics of perovskite and ilmenite concentrates. Izvestiya. Ferrous Metallurgy. 2024;67(1):27-36. https://doi.org/10.17073/0368-0797-2024-1-27-36