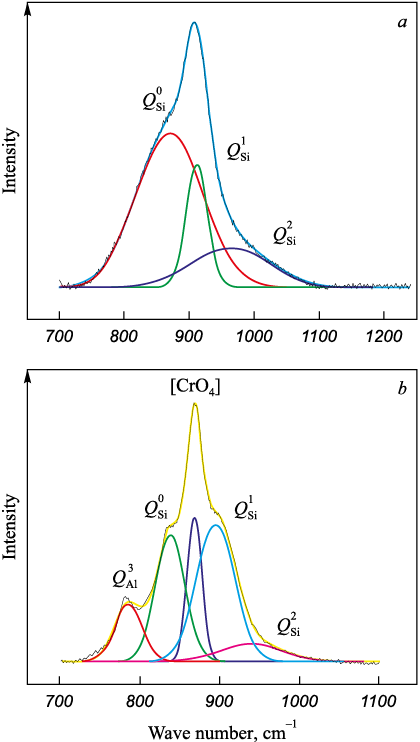Scroll to:
Influence of basicity on physical properties of slags of the СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 – 3 % Аl2O3 – 8 % МgO system
https://doi.org/10.17073/0368-0797-2023-6-743-749
Abstract
Influence of basicity on viscosity, crystallization onset temperature, phase composition, and structure of slags of the СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 – 3 % Аl2O3 – 8 % МgO system in the basicity range (B = CaO/SiO2 ) from 1.0 up to 2.5 was studied using vibrational viscometry, thermodynamic modeling, and Raman spectroscopy. It was established that the physical properties of slags depend on the balance of polymerization degree and phase composition. Acid slags with a basicity of 1.0 belong to the category of “long” slags and are characterized by an increased proportion of high-temperature phases up to 34.1 %. However, despite the fact that the proportion of high-temperature phases is 1.6 times higher compared to the proportion of low-temperature ones, they are characterized by a simpler silicate structure, providing a viscosity of no more than 0.25 Pa·s at a crystallization onset temperature of 1530 °C. An increase in basicity of slags of the studied oxide system (up to 2.5), along with an increase in the proportion of high-temperature phases (by almost 5.9 times), is accompanied by formation of a more complex silicate structure. The resulting four-coordination structural elements [CrO4] and [AlO4] are embedded in the silicate structure and complicate it, which increases the polymerization degree. Thus, at basicity of 2.5, due to a high proportion of high-temperature phases in the slag and development of polymerization process, slag crystallization onset temperature increases to 1700 °C and its viscosity reaches 1.0 Pa·s at a temperature of 1670 °C.
Keywords
For citations:
Babenko A.A., Shartdinov R.R., Upolovnikova A.G., Smetannikov A.N., Lobanov D.A., Dolmatov A.V. Influence of basicity on physical properties of slags of the СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 – 3 % Аl2O3 – 8 % МgO system. Izvestiya. Ferrous Metallurgy. 2023;66(6):743-749. https://doi.org/10.17073/0368-0797-2023-6-743-749
Introduction
In the contemporary field of low-carbon stainless steel production, argon-oxygen decarburization (AOD) technology predominates, entailing phases of oxidation and reduction. The reduction phase slags, rich in chromium oxide, present significant challenges in chromium reduction and steel desulfurization due to their high viscosity and refractory nature. To mitigate these issues, calcium fluoride is traditionally added as a fluxing agent during the reduction phase [1]. However, this addition poses drawbacks, including aggressive wear on the refractory lining, alterations in slag composition over time, and the formation of environmentally detrimental volatile fluorides [2]. Therefore, researchers have to find a way to replace it. One of the solutions can be the use of boron oxide. In response to these challenges, boron oxide emerges as a promising alternative, attributed to its beneficial impact on slag viscosity and crystallization temperature [3 ‒ 5]. Nonetheless, the specific influence of boron oxide on the physical properties of chromium-containing slags remains largely underexplored.
This study employs vibrational viscometry, thermodynamic modeling of phase composition (HSC Chemistry 6.12 (Outokumpu)), and Raman spectroscopy to investigate the effects of varying basicity (B = СaO/SiO2 ) from 1.0 to 2.5 – mirroring the composition at the commencement of the AOD process reduction period – on the viscosity η, crystallization onset temperature (tcr ), phase composition and structure of slags in the СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 –3 % Аl2O3 – 8 % МgO system [6].
Materials and methods
To study the physical properties of slags within the six-component oxide system СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 – 3 % Аl2O3 – 8 % МgO, experimental slags were synthesized with compositions detailed in Table 1.
Table 1. Composition of experimental slags
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
These slags were produced in a resistance furnace using molybdenum crucibles under an argon atmosphere, employing analytical-reagent grade oxides pre-calcinated at 800 °C (with B2O3 calcinated at 100 °C) for 2 to 3 h.
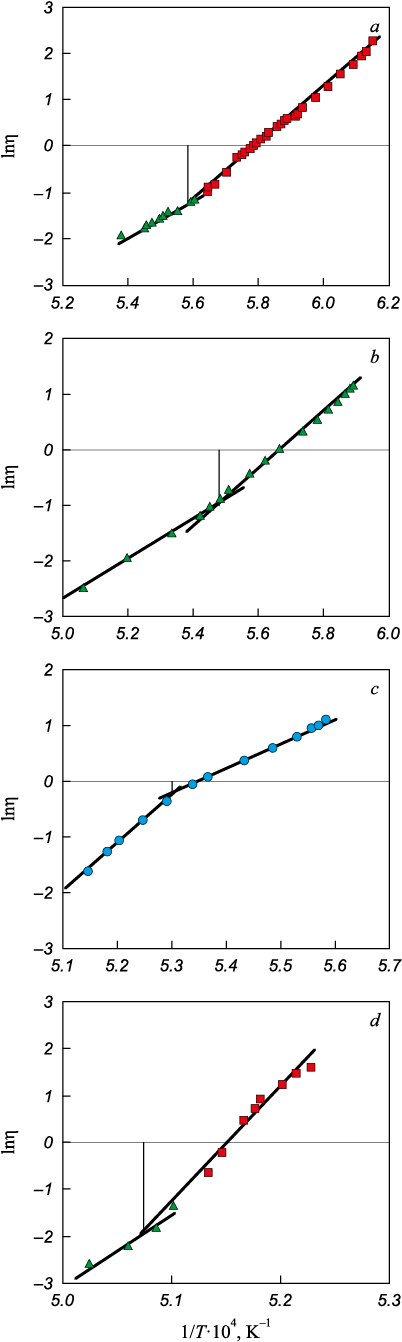
The viscosity measurements for these slags were conducted utilizing a vibrating viscometer [7] within molybdenum crucibles in an argon flow, with temperature monitoring achieved through a tungsten-rhenium thermocouple. The slags’ crystallization onset temperatures were ascertained based on Frenkel’s theory of viscous flow. This involved plotting graphs in the coordinates ln η – 1/T, with the crystallization temperature identified at the inflection point of these curves [8].
Thermodynamic modeling of the phase composition for the experimental slag samples was performed using the HSC Chemistry 6.12 software package (Outokumpu) [9].
The structure of slag samples was investigated using a Raman microscope spectrometer (U 1000) quipped with a 532 nm excitation wavelength laser. The acquired spectra span a wave number range of 200 to 1600 cm\(^‒\)1. The spectrum lines observed can be unequivocally linked to the vibrational movements of the molecules within the slag sample. An analysis of the slag’s structure is facilitated through examination of the oscillation frequency, as well as the intensity and contour of these spectrum lines [10].
Results and discussion
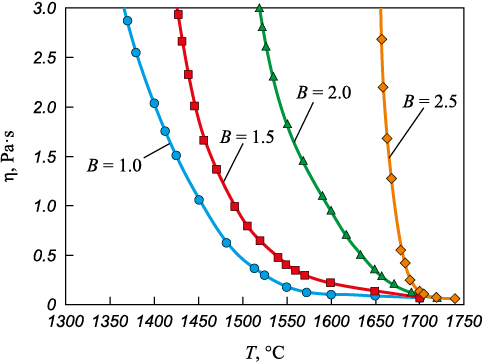
Fig. 1 illustrates the relationship between slag viscosity, temperature, and basicity. Fig. 2 presents these relationships within the coordinates ln η – 1/T, facilitating the determination of the crystallization temperature (Table 1).
Fig. 1. Dependence of viscosity on temperature
Fig. 2. Dependence of viscosity logarithm of (ln η) |
Table 2 outlines the phase composition modeling results for the slag samples tested. Based on their melting temperatures, all phases have been categorically divided into three groups: low-temperature (1130 ‒ 1280 °C), medium-temperature (1460 ‒ 1600 °C), and high-temperature (1710 ‒ 2852 °C).
Table 2. Phase composition of experimental slags at 1600 °C
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
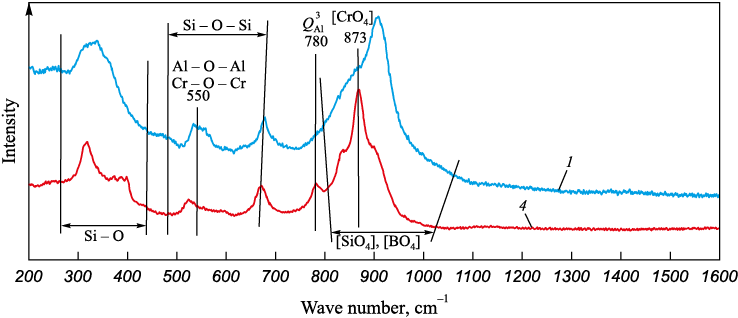
Raman spectroscopy results of the experimental slag samples, with basicities of 1.0 and 2.5 (slags 1 and 4, respectively) and a constant content of chromium oxide (18 %) and boron oxide (6.0 %), are depicted in Fig. 3. Table 3 correlates the wave numbers to the peaks of structural elements observed.
Fig. 3. Raman spectra of slags 1 and 4
Table 3. Correspondence of wave numbers and structures
|
Peaks within the wave number ranges of 470 to 660 and 250 to 400 cm\(^‒\)1 are associated with symmetric stretching and bending vibrations of Si – O – Si linkages. Peaks at 550 cm\(^‒\)1, found within these ranges, are attributed to Al – O – Al and Cr – O – Cr connections. As slag basicity increases, these peaks, including the Si – O – Si linkages, become less distinct.
Variations in the wave number region of 800 to 1200 cm\(^‒\)1 indicate that with an increase in basicity to 2.5, Raman spectrum peaks corresponding to [CrO4] and \(Q_{{\rm{Al}}}^3\) appear at wave numbers 873 and 780 cm\(^‒\)1. This suggests the presence of these structural components in slags with elevated basicity, recognized as slag polymerizers [14; 19].
Fig. 3 lacks peaks corresponding to three-coordination boron [BO3], indicating that within the slag structure, boron oxide is represented by four-coordination boron [ВO4 ]. The [BO4] tetrahedra tend to create bonds with silicon atoms, complicating the structure, but at the same time, reducing its uniformity and strength [20 ‒ 22]. Reduction in the slag viscosity when such oxide is used as a fluxing agent can be attributed to weakening of the structure and formation of low-melting compounds.
The polymerization degree of slag is primarily influenced by the high-frequency silicate region, spanning wave numbers 800 to 1200 cm\(^‒\)1, which correspond to [SiO4] tetrahedrons. To gain a more nuanced understanding of the slag’s structural intricacies, we performed deconvolution of the obtained Raman spectra using the Gaussian method [23] (Fig. 4). This process facilitated the representation of the slag’s polymerization degree through the quantification of the average number of bridging oxygen (BO) molecules, calculated by the formula:
| \[{\rm{BO}} = 0 \cdot Q_{{\rm{Si}}}^0 + 1 \cdot Q_{{\rm{Si}}}^1 + 2 \cdot Q_{{\rm{Si}}}^2 + 3 \cdot Q_{{\rm{Si}}}^3 + 4 \cdot Q_{{\rm{Si}}}^4,\] | (1) |
where \(Q_{{\rm{Si}}}^n\) is [SiO4 ] with n number of bridging oxygen.
Fig. 4. Results of deconvolution of slags 1 (а) and 4 (b) |
Calculations of the average amount of bridging oxygen (BO) are presented in Table 4.
Table 4. Fractions of silicate structural elements
| ||||||||||||||||||||||||||||
Acid slags with a basicity of 1.0 (Fig. 1, slag 1) categorized as “long” slags, are shown to possess a heightened proportion of high-temperature phases, reaching up to 34.1 % (Table 2). However, despite the fact that the proportion of high-temperature phases is 1.6 times higher compared to that of low-temperature phases, slags with a basicity of 1.0 have a simpler silicate structure. The average amount of bridging oxygen BO does not exceed 0.55, likely because chromium oxide behaves more like a base in the acidic slag environment [24; 25]. The depolymerizing impact on the silicon-oxygen lattice results in a majority (0.64) of the silicate structural elements being composed of [SiO4] units devoid of bridging oxygen. This simpler structure, particularly in slags with a basicity of 1.0, ensures relatively high fluidity at a crystallization temperature of 1530 °C, despite having a 1.6-fold greater proportion of high-temperature phases. At and above the crystallization temperature, the viscosity of the slag remains below 0.25 Pa·s.
As the basicity of the slags within this oxide system increases, the trend of a rising proportion of high-temperature phases and a declining proportion of low-temperature ones continues (as indicated in Table 2). For instance, a slag with a basicity of 2.5 (slag 4, Fig. 1) is classified as belonging to the “short” slags category (Table 2), with its high-temperature phase proportion escalating to 53.5 %. This increase is largely attributable to the phases 2CaO·SiO2 (21.9 %) and CaO·Cr2O3 (20.5 %), alongside a reduction in low-temperature phases to 9.1 %, due to diminished proportions of CaO·B2O3 and CaO·MgO·2SiO2 to 0.4 and 0.3 %, respectively. Concurrently, despite the enhanced basicity and the integration of the [BO4] structural element, the presence of chromium and aluminum oxides, acting as acidic oxides [14; 19; 20], leads to a heightened polymerization degree of the slag. The incorporation of four-coordination chromium [CrO4] and aluminum [AlO4] into the silicon-oxygen framework intensifies its complexity. Consequently, the average number of bridging oxygen (BO) rises to 0.73, predominantly because a significant portion (0.52) of the silicate structural elements consists of [SiO4] with one bridging oxygen. This intricate silicate structure, alongside an approximately 5.9-fold increase in the proportion of high-temperature phases compared to low-temperature ones, contributes to a rise in the crystallization temperature to 1700 °C and viscosity levels reaching 1.0 Pa·s or more at temperatures of 1670 °C and below.
Conclusions
Our research has revealed new details about how the basicity of slags in the СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 – 3 % Аl2O3 – 8 % МgO system impacts their phase composition, structure, viscosity, and the temperature at which they begin to crystallize.
We’ve found that the physical properties of slags hinge on the interaction between polymerization processes and their phase makeup:
– at a basicity level of 1.0, chromium oxide acts in a way that simplifies the slag’s structure, resulting in a bridging oxygen (BO) value of 0.55. This simple structure leads to a low viscosity of 0.25 Pa·s at the temperature where crystallization starts, which is 1530 °C, even though there’s a high presence of high-temperature phases;
– on the contrary, when the basicity reaches 2.5, the degree of polymerization in the slag increases (BO = 0.73). This is because Cr2O3 starts to show acidic properties, as seen by the formation of the [CrO4] structural unit in the slag. Along with this, there’s a significant increase in high-temperature phases, by about 1.57 times. This combination leads to a more complex structure, pushing the slag’s viscosity up to 1.0 Pa·s at 1670 °C and raising the temperature at which crystallization begins to 1700 °C.
References
1. Kalicka Z., Kawecka-Cebula E., Pytel K. Application of the Iida model for estimation of slag viscosity for Al2O3-Cr2O3-CaO-CaF2. Archives of Metallurgy and Materials. 2009;54(1):179–187.
2. Dyudkin D.A., Kisilenko V.V. Production of Steel. In 3 vols. Vol. 3. Extra-Furnace Metallurgy of Steel. Moscow: Teplotekhnik; 2010:544. (In Russ.).
3. Wang H.M., Li G.R., Li B., Zhang X.J., Yan Y.Q. Effect of B2O3 on melting temperature of cao-based ladle refining slag. Journal of Iron and Steel Research International. 2010;17(10):18–22. https://doi.org/10.1016/S1006-706X(10)60177-X
4. Wang, H.M., Zhang, T.W., Zhu, H., Yan, Y.Q., Zhao Y.N. Effect of B2O3 and CaF2 on viscosity of ladle refining slag. Advanced Materials Research. 2011;295–297:2647. https://doi.org/10.4028/www.scientific.net/AMR.295-297.2647
5. Babenko A.A., Shartdinov R.R., Upolovnikova A.G., Smetannikov A.N., Gulyakov V.S. Physical properties of slags of CaO – SiO2 – B2O3 system containing 15 % of Al2O3 and 8 % of MgO. Izvestiya. Ferrous Metallurgy. 2019;62(10):769–773. (In Russ.). https://doi.org/10.17073/0368-0797-2019-10-769-773
6. Tokovoi O.K. Argon-Oxygen Refining of Stainless Steel. Chelyabinsk: South Ural State University; 2015:250. (In Russ.).
7. Shtengel’meier S.V., Prusov V.A., Bogachev V.A. Improvement of the viscosity measurement technique with a vibrating viscometer. Zavodskaya laboratoriya. 1985;51(9):56–57. (In Russ.).
8. Voskoboinikov V.G., Dunaev N.E., Mikhalevich A.G, Kukhtin T.I., Shtengel’meier S.V. Properties of Liquid Blast Furnace Slags. Moscow: Metallurgiya; 1975:180. (In Russ.).
9. Roine A. HSC 6.0 Chemistry Reactions and Equilibrium Software with Extensive Thermochemical Database and Flowshut. Pori: Outokumpu research Oy; 2006:448.
10. Böcker J. Spektroskopie: Instrumentelle Analytik mit Atom- und Molekülspektrometrie. Würzburg: Vogel Communications Group GmbH & Co. KG; 1997:519. (In Germ.).
11. McMillan P. Structural studies of silicate glasses and melts-applications and limitations of Raman spectroscopy. American Mineralogist. 1984;69(6):622–644.
12. Matson D.W., Sharma S.K., Philpotts J.A. The structure of high-silica alkali-silicate glasses. A Raman spectroscopic in vestigation. Journal of Non-Crystalline Solids. 1983;58(2–3):323–352. https://doi.org/10.1016/0022-3093(83)90032-7
13. McMillan P.F., Poe B.T., Gillet P.H., Reynard B. A study of SiO2 glass and supercooled liquid to 1950 K via high-temperature Raman spectroscopy. Geochimica et Cosmochimica Acta. 2001;58(17):3653–3662. https://doi.org/10.1016/0016-7037(94)90156-2
14. Kim T.S., Park J.H. Structure-viscosity relationship of low-silica calcium aluminosilicate melts. ISIJ International. 2014;54(9):2031–2038. https://doi.org/10.2355/isijinternational.54.2031
15. Dines T.J., Inglis S. Raman spectroscopic study of supported chromium (VI) oxide catalysts. Physical Chemistry Chemical Physics. 2003;5(6):1320–1328. https://doi.org/10.1039/b211857b
16. Weckhuysen B.M., Wachs I.F. Raman spectroscopy of supported chromium oxide catalysts. Determination of chromium—oxygen bond distances and bond orders. Journal of the Chemical Society, Faraday Transactions. 1996;92(11):1969–1973. https://doi.org/10.1039/FT9969201969
17. Kim Y., Morita K. Relationship between molten oxide structure and thermal conductivity in the CaO–SiO2–B2O3 system. ISIJ International. 2014;54(9):2077–2083. https://doi.org/10.2355/isijinternational.54.2077
18. Cochain B., Neuville D.R., Henderson G.S., McCammon C.A., Pinet O., Richet P. Effects of the iron content and redox state on the structure of sodium borosilicate glasses: A Raman, Mössbauer and boron K‐Edge XANES spectroscopy study. Journal of the American Ceramic Society.2012;95(3):962–971. https://doi.org/10.1111/j.1551-2916.2011.05020.x
19. Li Q., Gao J., Zhang Y., An Z., Guo Z. Viscosity measurement and structure analysis of Cr2O3-bearing CaO-SiO2-MgO-Al2O3 slags. Metallurgical and Materials Transactions B. 2017;48:346–356. https://doi.org/10.1007/s11663-016-0858-8
20. Xu R.Z., Zhang J.L., Wang Z.Y., Jiao K.X. Influence of Cr2O3 and B2O3 on viscosity and structure of high alumina slag. Steel Research International. 2017;88(4):1600241. https://doi.org/10.1002/srin.201600241
21. Sun Y., Zhang Z. Structural roles of boron and silicon in the CaO-SiO2-B2O3 glasses using FTIR, Raman, and NMR spectroscopy. Metallurgical and Materials Transactions B. 2015;46:1549–1554. https://doi.org/10.1007/s11663-015-0374-2
22. Cai Z., Song B., Li L., Liu Zh., Cui X. Effects of B2O3 on viscosity, structure, and crystallization of mold fluxes for casting rare earth alloyed steels. Metals. 2018;8(10):737. https://doi.org/doi:10.3390/met8100737
23. Mysen B.O., Virgo D., Scarfe C.M. Relations between the anionic structure and viscosity of silicate melts – a Raman spectroscopic study. American Mineralogist. 1980;65(7): 690–710.
24. Forsbacka L., Holappa L., Kondratiev A., Jak E. Experimental study and modelling of viscosity of chromium containing slags. Steel Research International. 2007;78(9):676–684. http://dx.doi.org/10.1002/srin.200706269
25. Wu T., Zhang Y., Yuan F., An Z. Effects of the Cr2O3 content on the viscosity of CaO-SiO2-10 Pct Al2O3-Cr2O3 quaternary slag. Metallurgical and Materials Transactions B. 2018;49:1719–1731. https://doi.org/10.1007/s11663-018-1258-z
26.
About the Authors
A. A. BabenkoRussian Federation
Anatolii A. Babenko, Dr. Sci. (Eng.), Prof., Chief Researcher
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
R. R. Shartdinov
Russian Federation
Ruslan R. Shartdinov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. G. Upolovnikova
Russian Federation
Alena G. Upolovnikova, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. N. Smetannikov
Russian Federation
Artem N. Smetannikov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
D. A. Lobanov
Russian Federation
Daniil A. Lobanov, Cand. Sci. (Eng.), Research Associate
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. V. Dolmatov
Russian Federation
Aleksei V. Dolmatov, Cand. Sci. (Chem.), Senior Researcher of the Laboratory of Metallurgical Melts
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Babenko A.A., Shartdinov R.R., Upolovnikova A.G., Smetannikov A.N., Lobanov D.A., Dolmatov A.V. Influence of basicity on physical properties of slags of the СаО – SiO2 – 18 % Cr2O3 – 6 % B2O3 – 3 % Аl2O3 – 8 % МgO system. Izvestiya. Ferrous Metallurgy. 2023;66(6):743-749. https://doi.org/10.17073/0368-0797-2023-6-743-749