Scroll to:
Structural changes in the melt of a heat-resistant nickel alloy as phase transition of the second order
https://doi.org/10.17073/0368-0797-2023-5-564-570
Abstract
Information about the behavior of melts of the high-temperature nickel alloys is the basis for creating new smelting technologies that significantly increase the service properties of metal products, as well as solve a number of technological problems. The results of numerous studies indicate structural changes occurring in various metal melts under the influence of temperature and time. For many years, there has been a scientific discussion about the nature of these phenomena, and a common opinion was formulated on a number of issues. Structural changes in metallic liquids are presented as a second-order phase transition, where a liquid of higher density is replaced by a liquid of lower density. These transformations in the structures of liquid metals are called liquid-liquid transition (LLT). Studies of the structure-sensitive properties of melts of the heat-resistant nickel alloys also reveal structural changes that irreversibly transform the melt into a microhomogeneous state. The research results presented in this article confirmed that structural changes in melts of the high-temperature nickel alloys are also a second-order phase transition, as evidenced by the breakage of atomic microgroups, uniform redistribution of alloying elements, and the formation of new clusters characterized by smaller sizes and greater chemical homogeneity. Therefore, these changes can be characterized as LLT, while this does not contradict the previously substantiated quasi-crystalline model of the microinhomogeneous state of liquid heat-resistant nickel alloys.
Keywords
For citations:
Mil’der O.B., Tarasov D.A., Tyagunov A.G., Tsepelev V.S., V’yukhin V.V., Levonyan A.L., Anoshina O.V. Structural changes in the melt of a heat-resistant nickel alloy as phase transition of the second order. Izvestiya. Ferrous Metallurgy. 2023;66(5):564-570. https://doi.org/10.17073/0368-0797-2023-5-564-570
Introduction
An important aspect in enhancing the properties of metal products rests in the preparation of the melts for crystallization, contingent upon attaining an equilibrium state within a broad temperature range. The existence of a non-equilibrium melt, slightly above the liquidus temperature, can be explained by the residual presence of solid structures. This phenomenon is comprehended through the lens of a quasicrystalline model portraying a micro-inhomogeneous state, depicted as an array of atomic microgroups (clusters) of varying sizes, coupled with an irregular distribution of alloying elements. As the temperature of the melt rises during heating, the structural transformations take place. Consequently, the melt attains a state of equilibrium, characterized by micro-homogeneity, sustained until the onset of crystallization. This transformation yields advantageous casting structures and substantially enhances the material’s functional properties. This thermal treatment of melt finds extensive industrial utility and is denoted as thermal time treatment (TTT) or high-temperature melt treatment (HTMT) [1].
Multi-component heat-resistant nickel compositions are used in manufacturing the most critical components of gas turbine engines designed to operate under high- temperature and tensile stress conditions. The chemical composition comprises up to 22 alloying elements: C, Cr, Co, Mo, W, Al, Ti, Nb, B, Fe, Y, Zr, Ta, Re, Ru, V, Ce, La, Mn, Mg, Hf, Si, and may also contain challenging-to-remove impurities such as S, Si, P, and dissolved gases O, N. However, at the metallurgical production stage, several challenges emerge, including defects, low yield, and complexities in utilizing waste materials. The application of HTMT for heat-resistant nickel alloys has substantially addressed these issues and notably improved the quality of metal products [2].
The development of HTMT procedures for heat-resistant nickel alloys is rooted in an in-depth examination of structural changes occurring in melts during heating. A proposed quasicrystalline model delineates a micro-inhomogeneous state for melts of heat-resistant nickel alloys [1]: liquid heat-resistant nickel alloys comprise atomic microgroups exhibiting a stoichiometric composition akin to the primary strengthening γ′-phase Ni3(Al, Ti). The act of heating or prolonged isothermal holding of a metallic liquid facilitates the transformation of melts into a homogeneous and microhomogeneous state across an extensive temperature range. This transformation is irreversible and persists until the initiation of solid structure formation. For most investigated metallic materials, such structural changes represent a second-order phase transition, known as a liquid–liquid transition (LLT) [3; 4]. Post-restructuring, a stable metallic liquid emerges, comprising homogeneous atomic microgroups characterized by smaller radii, altered interatomic distances, or modified coordination numbers [3; 4].
Experimental evidence supports the structural modifications observed in the metal melts of heat-resistant nickel alloys [3 – 5]. Given the ongoing debate surrounding the mechanism underlying these changes in nickel-based alloys, this study aims to ascertain whether the structural alterations occurring during the heating of nickel compositions align with a second-order phase transition.
Experimental
The heat-resistant nickel alloy ZhS6U was selected as the test material due to its frequent industrial usage. The melting composition is detailed in Table.
Melting chemical composition of ZhS6U heat-resistant nickel alloy sample, at. %
|
According to the Landau phenomenological theory [6], second-order phase transitions are characterized by the continuity of state changes without any release or absorption of latent energy.
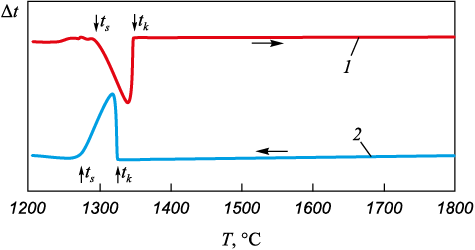
Differential Thermal Analysis (DTA) stands as the primary method for detecting the presence of latent energy release or absorption in a process. Fig. 1 displays DTA curves acquired during the heating (1) and subsequent cooling (2) of the ZhS6U alloy. These curve patterns are typical for a majority of heat-resistant nickel alloy grades.
Fig. 1. DTA curves of ZhS6U alloy: |
The arrows in the figures indicate the solidus temperature (tS) and liquidus temperature (tL). Within the temperature range of 1250 to 1350 °C, distinct thermal effects related to both the melting (1) and subsequent crystallization (2) of the studied alloy were identified. Above the liquidus temperature, the DTA curves exhibit a horizontal line, indicating a complete absence of heat absorption or release over an extended temperature range. The experimental findings strongly suggest the absence of latent heat during the structural changes in the liquid heat-resistant alloy.
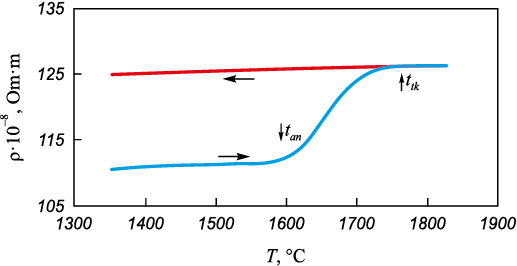
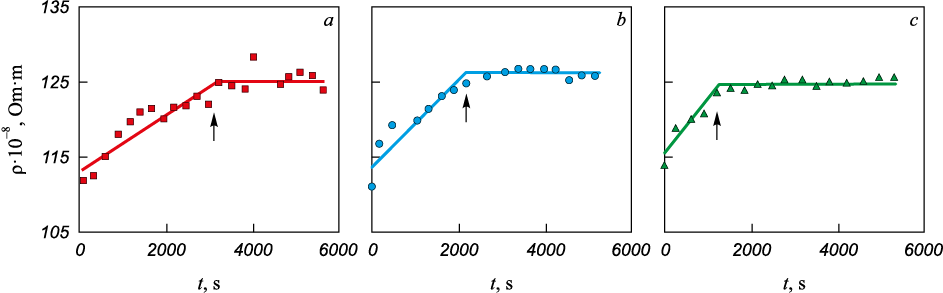
For this investigation, the electrical resistivity method was chosen due to its high sensitivity in studying structural alterations in melts of heat-resistant nickel alloys [2]. Further details regarding this technique are available in [7]. Polytherms ρ = f (t) (Fig. 2) and isotherms ρ = f (τ) (Fig. 3) of the considered alloy were analyzed.
Fig. 2. Electrical resistivity polytherms of ZhS6U alloy
Fig. 3. Electrical resistivity isotherms of ZhS6U melt obtained at temperatures, °С: |
The curves ρ = f (t) obtained during both heating and subsequent cooling of the ZhS6U melt exhibit a characteristic pattern observed in most liquid heat-resistant nickel compositions [2; 5]. The heating polytherm reveals a non-monotonic change in electrical resistivity, featuring specific points denoted as tan and tk . Between these points, an interval with an anomalous increase in electrical resistivity is observed. Another characteristic of electrical resistivity polytherms in heat-resistant nickel alloys is the phenomenon of hysteresis, showcasing a discrepancy between the heating and cooling branches.
To discern the nature of structural alterations occurring in the ZhS6U melt within the temperature range below tk , measurements of electrical resistivity were conducted during isothermal holding at temperatures of 1417, 1448, and 1479 °C. The observed electrical resistance was recorded at intervals of 300 s (5 min).
Based on the experimental findings, it was observed that during the duration of isothermal exposures, the electrical resistivity consistently rose until reaching a peak (Fig. 3), following which its sensitivity to time diminished. Moreover, higher holding temperatures resulted in a shorter duration for the melt to reach its peak resistivity.
Results and discussion
The behavior of the ρ = f (t) function and the positioning of points tan and tk explanation through the quasi-chemical model of the micro-inhomogeneous state in heat-resistant nickel alloys [1]: upon immediate melting, the structure of the ZhS6U melt exhibits homogeneity but retains a micro-inhomogeneous nature. It comprises dynamic clusters of varying sizes and uneven distribution of atoms from the alloy’s chemical composition. With rising temperatures, a transition occurs toward a state of enhanced homogeneity and microhomogeneity, characterized by more uniform sizes and compositions of atomic associations. The cooling polytherms of samples preheated to temperatures exceeding tk exhibit linearity, signifying that structural changes persist during cooling across an extensive temperature range. This suggests that the melt approaches a more equilibrium state before the onset of solidification.
The Drude’s theory proposes an equation defining the electrical conductivity of metals as follows
| \[\frac{1}{\rho } = \sigma = \frac{{n{e^2}{\tau _m}}}{m},\] | (1) |
where n is the concentration of all electrons per unit volume (considered as the concentration of conduction electrons in contemporary models), while τm is the average free travel time.
In modern models, the equation retains its formal structure; however, the interpretation of the included parameters evolves [8]. Notably, the only parameter correlated with the nonlinear rise in electrical resistivity is the average free travel time. This nonlinear increase in electrical resistivity (equivalently, decrease in electrical conductivity) is attributed to a heightened presence of scattering centers.
The phenomenon of hysteresis in the physical properties of melts (Fig. 2), characterized by a discrepancy between the forward and reverse branches, has been extensively detailed by several researchers [9 – 12]. These studies emphasize the irreversibility of changes occurring in the melt over a broad temperature range.
Analysis of the experimental data illustrated in Fig. 3 reveals that the time constant diminishes with escalating temperatures during isothermal holding, indicating a relationship described by θ = f (t). Additionally, an empirical regularity emerged:
| (t – tliq )θ = const, | (2) |
where t is the temperature of isothermal holding, while tliq is the liquidus point.
By formally approaching the time constant towards zero, we can approximate the temperature at which the vast majority of clusters undergo dissolution, signifying the temperature tk corresponding to the transition of the metallic liquid into a microhomogeneous state.
Consequently, structural transformations within the metallic liquid transpire not only during the process of heating to temperature tk , but also as a consequence of prolonged time exposures. In essence, the decay of clusters occurs not solely due to alterations in the system’s thermodynamic parameters (polytherm) but also when these parameters remain fixed (isotherm). This observation underscores the continuum of the ongoing process, a characteristic indicative of a second-order phase transition, as previously noted.
Parallel results concerning relaxation during extended exposures have been reported by other researchers [13 – 15].
Given that the second-order LLT phase transition exhibits both thermodynamic and structural characteristics, the provided thermodynamic rationale allows us to infer the mechanism of structural modifications in the ZhS6U melt. Upon immediate melting, the melt assumes a micro-inhomogeneous state, characterized by clusters of varying sizes and an uneven distribution of chemical elements. Subsequent heating and/or isothermal holding induce a second-order LLT phase transition within the melt: the original structure of the metallic liquid is replaced by a similar structure possessing a lower density. This transition involves the breakdown of cluster formations, a uniform redistribution of atoms, and the formation of new clusters with smaller sizes. The rise in electrical resistivity also indicates an increase in the number of atomic microgroups. These structural changes are irreversible, as evidenced by the disparity between the cooling polytherm and the heating polytherm. The resultant structural state demonstrates stability and microhomogeneity, validated by the absence of extremities in the cooling polytherm.
These observations align with the findings of authors in [16], who also affirm that the stability of cluster sizes in each phase region signifies a microhomogeneous state.
Moreover, the works by authors in [17 – 20] echo similar conclusions. They further highlight a notable sharp decrease in lattice parameter and coordination number with increasing temperature, contributing significantly to the observed increase in electrical resistivity.
Conclusions
Investigations on the heat-resistant nickel alloy ZhS6U reveal that when subjected to heating or isothermal holding, the alloy undergoes structural transformations, transitioning into a microhomogeneous state across an extensive temperature range. The delineated boundaries of these structural alterations on the electrical resistivity polytherms of the melt are denoted as tan and tk indicating irreversible changes.
The experimentally derived time constant, describing the process of structural modifications in the nickel-based melt during fixed thermodynamic parameters (isothermal holding), substantiates that these changes correspond to a second-order LLT phase transition.
The presented thermodynamic evidence elucidates the nature of structural changes in the melt of the heat-resistant nickel alloy, indicating a thermal-temporal effect referred to as the liquid-liquid transformation (LLT). Prior to LLT, the melt comprises clusters of varying sizes and configurations of atoms. During the LLT phase, atomic microgroups disintegrate, leading to a uniform redistribution of alloying elements, consequently forming new clusters characterized by reduced sizes and enhanced chemical homogeneity.
These findings align with the quasicrystalline theory of the microinhomogeneous state of metallic liquids, serving as an extension and not contradicting its principles.
References
1. Baum B.A. Metallic Liquids. Moscow: Nauka; 1979:120. (In Russ.).
2. Efimov V.E. Influence of temperature-time treatment of Ni3Al based intermetallic alloys on their phase composition and heat resistance. In: Aviation Materials and Technologies. High Heat-Resistant Materials for Modern and Advanced Gas Turbine Engines and Advanced Technologies for their Production. Moscow: VIAM; 2003:79–92. (In Russ.).
3. Nikolaev B.V., Tyagunov G.V., Baum B.A., Baryshev E.E., Larionov V.N., Khlystov V.N., Buler T.P., Pechatnikov M.I. Influence of melt preparation on structure and properties of Ni3Al based intermetallic alloy. Metally. 1991;(1):104–110. (In Russ.).
4. Zu F.-Q. Temperature-induced liquid-liquid transition in metallic melts: A brief review on the new physical phenomenon. Metals. 2015;5(1):395–417. https://doi.org/10.3390/met5010395
5. Buntushkin V.P., Efimov V.E., Nikolaev V.V. Influence of mycoadditives on critical melt temperature and heat resistance of a casting alloy based on Ni3Al intermetallic compound. Metally. 1995;(3):60-69. (In Russ.).
6. Landau L.D., Lifshits E.M. Theoretical Physics. Statistical Physics. Part I. Vol. V. Moscow: Nauka; 1976:584. (In Russ.).
7. Tyagunov G.V., Baum B.A., Tsepelev V.S., Tyagunov A.G. Measurement of resistivity by the method of rotating magnetic field. Zavodskaya laboratoriya. Diagnostika materialov. 2003;69(2):35–37. (In Russ.).
8. Blakemore J.S. Solid State Physics. Cambridge University Press;1985, 506. https://doi.org/10.1017/CBO9781139167871
9. Tsepelev V.S., Konashkov V.V., Baum B.A. Properties of Metal Melts. Part 1. Yekaterinburg: USTU-UPI; 2008:358. (In Russ.).
10. Tsepelev V.S., Konashkov V.V., Baum B.A. Properties of Metal Melts. Part 2. Yekaterinburg. USTU-UPI; 2008:383. (In Russ.).
11. Ri Kh., Ri E.Kh., Khimukhin S.N., Ri V.E., Zernova T.S., Knyazev G.A. Influence of thermal treatment on structure formation and properties of aluminum alloys. Vestnik TOGU. 2013;(2(29)):137–144. (In Russ.).
12. Li Y., Chen W., Dong B., Zhou S. Effects of metalloid content on viscosity of Fe-Si-B-P-C alloy melt. Journal of Non-Crystalline Solids. 2018;490:31–34. https://doi.org/10.1016/j.jnoncrysol.2018.03.042
13. Lad’yanov V.I., Lagunov S.V., Pakhomov S.V. On oscillating relaxation processes in nonequilibrium metal melts after melting. Metally. 1998;(5):20–23. (In Russ.).
14. Vasin M.G., Lad’yanov V.I., Bovin V.P. On the mechanism of nonmonotonic relaxation processes in metal melts. Metally. 2000;(5):27–32. (In Russ.).
15. Kolotukhin E.V., Tyagunov G.V., Nikolaev B.V., Baum B.A. On kinetic mode of relaxation of the structure of a multicomponent metal melt. Journal of Physical Chemistry. 1989;63(4):1118–1121. (In Russ.).
16. Wang L., Bian X.F., Liu J.T. Discontinuous structural phase transition of liquid metal and alloys. Physics Letters A. 2004;326(5-6):429–435. https://doi.org/10.1016/j.physleta.2004.04.052
17. Borovykh M.A., Chikova O.A., Tsepelev V.S., V’yukhin V.V. Effect of heat treatment conditions on electrical resistivity of 35KhGF molten steel. Izvestiya. Ferrous Metallurgy. 2018;61(3):237–243. (In Russ.). https://doi.org/10.17073/0368-0797-2018-3-237-243
18. Popel P.S., Sidorov V.E. Microheterogeneity of liquid metallic solutions and its influence on the structure and properties of rapidly quenched alloys. Materials Science and Engineering: A. 1997:226–228:237–244. https://doi.org/10.1016/S0921-5093(96)10624-9
19. Su H., Wang H., Zhang J., Guo M., Liu L., Fu H. Influence of melt superheating treatment on solidification characteristics and rupture life of a third-generation Ni-based single-crystal superalloy. Metallurgical and Materials Transactions B. 2018;49(4):1537–1546. https://doi.org/10.1007/s11663-018-1256-1
20. Wang L.N., Sun X.F., Guan H.R. Effect of melt heat treatment on MC carbide formation in nickel-based superalloy K465. Results in Physics. 2017;7:2111–2117. https://doi.org/10.1016/j.rinp.2017.06.020
About the Authors
O. B. Mil’derRussian Federation
Oleg B. Mil’der, Cand. Sci. (Phys.–Math.), Assist. Prof.
19 Mira Str., Yekaterinburg 620002, Russian Federation
D. A. Tarasov
Russian Federation
Dmitrii A. Tarasov, Cand. Sci. (Eng.), Assist. Prof.
19 Mira Str., Yekaterinburg 620002, Russian Federation
A. G. Tyagunov
Russian Federation
Andrei G. Tyagunov, Cand. Sci. (Eng.), Assist. Prof.
19 Mira Str., Yekaterinburg 620002, Russian Federation
V. S. Tsepelev
Russian Federation
Vladimir S. Tsepelev, Dr. Sci. (Eng.), Prof., Director of the Research Center of Physics of Metallic Liquids
19 Mira Str., Yekaterinburg 620002, Russian Federation
V. V. V’yukhin
Russian Federation
Vladimir V. V’yukhin, Research Associate of the Research Center of Physics of Metallic Liquids
19 Mira Str., Yekaterinburg 620002, Russian Federation
A. L. Levonyan
Armenia
Aik L. Levonyan, Cand. Sci. (Eng.), Assist. Prof.
105 Teryan Str., Yerevan 0009, Armenia
O. V. Anoshina
Russian Federation
Ol’ga V. Anoshina, Cand. Sci. (Phys.–Math.), Assist. Prof.
11 Mashinostroitelei Str., Yekaterinburg 620012, Russian Federation
Review
For citations:
Mil’der O.B., Tarasov D.A., Tyagunov A.G., Tsepelev V.S., V’yukhin V.V., Levonyan A.L., Anoshina O.V. Structural changes in the melt of a heat-resistant nickel alloy as phase transition of the second order. Izvestiya. Ferrous Metallurgy. 2023;66(5):564-570. https://doi.org/10.17073/0368-0797-2023-5-564-570