Scroll to:
Effect of morphology and volume fraction of δ-ferrite on hydrogen embrittlement of stainless steel produced by electron beam additive manufacturing
https://doi.org/10.17073/0368-0797-2023-4-434-441
Abstract
The authors studied the influence of volume fraction and morphology of δ-ferrite on hydrogen embrittlement of austenitic stainless steel 08Kh19N9T obtained by electron beam additive manufacturing. It is experimentally shown that in additively-manufactured samples, long lamellae of δ-ferrite form a dense “net” of interphase boundaries (austenite/δ-ferrite, the volume fraction of the δ-phase is 20 %) and contribute to the hydrogen accumulation. Also, being the “easy” ways for the diffusion of hydrogen atoms, the dendritic lamellae of ferrite provide hydrogen transport deep into the samples. Post-production solid-solution treatment (at T = 1100 °C, 1 h) leads to a significant decrease in the fraction of δ-ferrite in steel (up to 5 %) and partial dissolution of dendritic lamellae. A decrease in the volume fraction of ferrite and a change in its morphology hinder the diffusion of hydrogen deep into the samples and its accumulation during electrolytic hydrogen-charging and subsequent deformation. It contributes to a decrease in the total concentration of hydrogen dissolved in the steel samples. Despite the lower concentration of dissolved hydrogen in the solid-solution treated samples, the solid-solution strengthening by hydrogen atoms is higher (\(\Delta \sigma _{0.2}^{\rm{H}}\) = 73 MPa) than for the initial samples with a high content of δ-ferrite (\(\Delta \sigma _{0.2}^{\rm{H}}\) = 55 MPa). The solid-solution treated samples are characterized by a smaller thickness of the brittle surface hydrogen-charged layer and a lower hydrogen embrittlement index compared to the post-produced samples (DH = 55 ± 12 µm, IH = 32 % for initial samples and DH = 29 ± 7 µm, IH = 24 % for samples after post-production solid-solution treatment).
Keywords
For citations:
Panchenko M.Yu., Reunova K.A., Nifontov A.S., Kolubaev E.A., Astafurova E.G. Effect of morphology and volume fraction of δ-ferrite on hydrogen embrittlement of stainless steel produced by electron beam additive manufacturing. Izvestiya. Ferrous Metallurgy. 2023;66(4):434-441. https://doi.org/10.17073/0368-0797-2023-4-434-441
Introduction
Additive manufacturing (AM) represents a rapidly advancing domain encompassing both science and industry. It introduces a transformative approach to crafting intricate components and parts for mechanisms characterized by intricate configurations, a feat previously unattainable using conventional manufacturing techniques. The application of additive technologies holds significant potential within the burgeoning sphere of hydrogen energy. This encompasses endeavors related to hydrogen storage, transportation, and the fabrication of essential components like hydrogen fuel cells and vehicles. Stable austenitic stainless steels exhibit the highest resistance to hydrogen’s adverse effects when compared to other steel categories [1; 2]. Their favorable weldability and moderate cost make them an appealing choice for utilization as foundational materials within additive technologies [3; 4]. Nevertheless, when devising components intended for operation in aggressive hydrogen-rich environments, it becomes imperative to consider the unique microstructural attributes intrinsic to this steel category that manifest during the AM process. These attributes take into account the anisotropic nature of grain structure [5; 6] and formation of the secondary phases [7 – 9].
Currently, the subject of hydrogen embrittlement in steel samples produced through AM techniques remains inadequately explored in published literature, with occasional inconsistencies in the available data. The study [10] revealed that austenitic steel AISI 304L, fabricated using laser powder AM technology, is more resistant to hydrogen embrittlement compared to conventionally obtained rolled steel. This enhanced resistance is attributed to the development of a stable austenitic phase that is resistant to phase transitions, as well as the distinctive texture of the steel brought about during powder sintering. Research presented in [11] demonstrated the heightened vulnerability of martensitic aging steel produced through selective laser melting to hydrogen embrittlement. Stainless steel 17-4 PH, featuring a coarse crystalline ferritic structure and manufactured via laser additive technologies, is more prone to the adverse effects of hydrogen than its cast counterpart [12]. Conversely, for ferritic steel 09G2S, additively produced steel using the electron beam AM method exhibits a weaker susceptibility to hydrogen embrittlement than its cast equivalent [13].
In comprehending the reasons behind the degradation of mechanical properties induced by hydrogen in additively manufactured austenitic steels – known for their resistance to hydrogen embrittlement – grasping the role of key microstructural aspects in these processes becomes crucial. Particularly, samples of austenitic steels generated through electron beam AM methods exhibit distinctive characteristics: an anisotropic grain structure and a notable proportion of the δ-phase (approximately 20 %) [6 – 8]. This study aims to strategically manipulate the morphology and volume fraction of δ-ferrite within 08Kh19N9T steel samples produced via electron beam additive manufacturing (EBAM), while keeping other structural parameters (primarily the size of austenite grains) unchanged [6].
The objective of this research is to establish patterns concerning the hydrogen embrittlement of austenitic steel 08Kh19N9T manufactured using the EBAM technique, contingent on the morphology and content of δ-ferrite in its structure.
Experimental methods
For the EBAM process, we utilized an industrial wire composed of austenitic stainless steel (ASS) 08Kh19N9T, with the following chemical composition (wt. %): Cr 17.7; Ni 9.7; Mn 1.1; Ti 0.8; Si 0.6; C 0.08; with iron constituting the remainder (wire diameter 1.2 mm). The electron beam additive manufacturing was executed within a vacuum chamber, employing the subsequent parameters: accelerating voltage of 30 kV, wire feed speed set at 180 mm/min, beam sweep spanning 45×45 mm, scanning frequency of 1 kHz, and electron beam current of 45 mA. The accumulation of material layers transpired atop an austenitic steel substrate, resulting in a final geometric configuration of the steel billet measuring 110×6×30 mm.
For the purpose of uniaxial static tension tests, flat samples resembling double blades were fashioned, with the working part dimensions at 12×3×1.5 mm. These samples were extracted from an additively manufactured steel wall. A portion of the samples were assessed in their initial state – directly following additive growth (AM-ASS) – while another subset underwent annealing at 1100°C for 1 hour, succeeded by water quenching (AM-ASS + HT). Surface treatment encompassed mechanical grinding and electrolytic polishing using a solution of 25 g CrO3 and 200 ml H3PO4 .
Microstructural analysis of the obtained samples was conducted using optical (OM) and transmission electron microscopy (TEM), utilizing Altami MET 1C and Jeol JEM 2100 microscopes, respectively. The magnetic phase analysis (MPA) technique was employed, specifically with an MVP-3 ferritometer (Kropus, Russia) to determine the volume fraction of the δ phase (Vδ ).
Electrolytic hydrogenation was executed in a 3 % NaCl aqueous solution containing 3 g/L NH4SCN. This procedure spanned 50 h at a current density of 50 mA/cm2. Thermal desorption spectroscopy (TDS) investigations – centered on evaluating the rate of hydrogen liberation from specimens during gradual heating – were conducted using an automated Gas Reaction Controller LBP system (Advanced Materials Research, USA). This was executed with a heating rate of 360 °C/h, within a temperature span ranging from 25 to 400 °C. Tensile tests were carried out at room temperature, maintaining an initial strain rate of 5·10\(^-\)4 s\(^-\)1. The testing was performed employing an LFM-125 universal electromechanical testing machine (Walter+Bai AG, Switzerland). Subsequently, the fracture surfaces of the samples were meticulously analyzed using a LEO EVO 50 scanning electron microscope (SEM) (Zeiss, Germany). To estimate the thickness of the brittle hydrogen-induced layer, SEM images acquired from the fracture surfaces of hydrogenated samples were employed. The secant method was utilized for this purpose, wherein the secants were oriented perpendicular to the hydrogenated layer.
Results and discussion
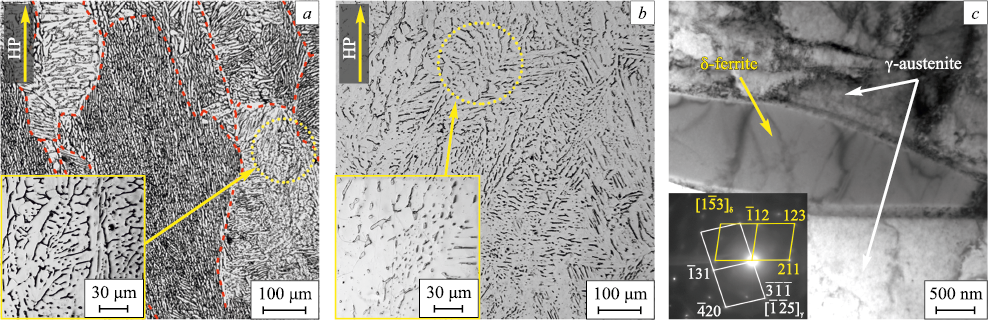
Fig. 1 presents optical microscopy (OM) and transmission electron microscopy (TEM) images of samples produced via the EBAM process. Both categories of samples exhibit a dual-phase (γ + δ) configuration. Notably, the δ-ferrite lamellae emergence of the δ phase within the structure of additively manufactured samples is attributed to distinct characteristics of the EBAM process, encompassing the intricate, multistage thermal history of layers and the overall billet, as well as the diminution of nickel content within the melt, among other factors [7; 9; 14]. Subsequent heat treatment (1100 °C, 1 h) induces a reduction in the δ-ferrite content while simultaneously altering its morphology – a phenomenon exhaustively elucidated in [6]. In specific, the heat treatment results in the formation of a dendritic structure of δ-ferrite, characterized by an average lamella thickness of 0.8 ± 0.4 µm within the AM-ASS samples. Following heat treatment, prolonged continuous branches (lamellae) of δ-ferrite experience partial dissolution, giving rise to non-equiaxed δ-phase grains (particles). These new grains exhibit an average thickness and length of 1.3 ± 0.5 and 6.2 ± 3.1 μm, respectively (Fig. 1, b). Consequently, the volume fraction of ferrite diminishes from 20 % to 5 % after the heat treatment, while the grain structure of the principal phase (austenite) remains unaffected [6]. Thus, the heat treatment exclusively influences the morphology and volume fraction of the ferrite phase, maintaining all other structural parameters unaltered.
Fig. 1. OM images (a, b) and SEM image (c) of the samples of additive manufactured austenic |
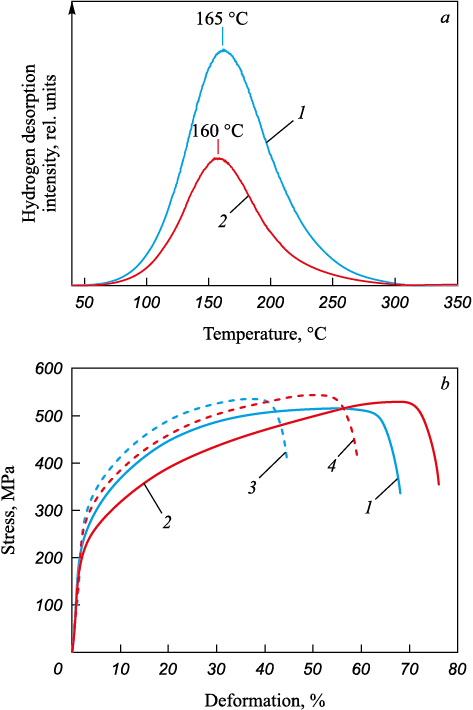
For hydrogenated AM-ASS and AM-ASS + HT samples, their thermal desorption (TDS) spectra depict a sole peak at Tmax = 160 – 165 °C (Fig. 2, a). This low-temperature peak corresponds to the release of hydrogen atoms from the crystal lattice and various weak, reversible traps possessing low activation energies. These encompass grain boundaries, interfacial (austenite – δ-ferrite) boundaries, dislocations, and similar features [15; 16]. The peak intensity for AM-ASS samples surpasses that of AM-ASS + HT samples, wherein the latter category bears a reduced proportion of the δ-phase. Hence, under analogous saturation conditions, a lesser amount of hydrogen dissolves within AM-ASS + HT samples. This disparity might arise due to the diminished presence of interphase boundaries in the annealed samples, which are capable of adsorbing hydrogen atoms. Given that the diffusion coefficient of hydrogen in δ-ferrite is significantly higher and its solubility lower than in the austenite phase [17; 18], the elevated volume fraction of the δ-phase, along with its morphology characterized by extended continuous lamellae in AM-ASS samples, expedites the profound penetration of hydrogen atoms into the samples during saturation. Put differently, the δ-ferrite branches serve as preferential conduits for the inward transport of hydrogen atoms, contributing to a more effective accumulation of hydrogen within the material (primarily within austenite). This proposition also correlates with the observation of the TDS peak shifting towards higher temperatures for AM-ASS samples in comparison to the peak for AM-ASS + HT samples. This shift could be attributed to a deeper saturation of the samples with hydrogen as the ferrite proportion increases.
Fig. 2. Thermodesorbtion spectra (a) and stress–strain diagrams |
Fig. 2, b delineates stress-strain diagrams in engineering coordinates for AM-ASS and AM-ASS + HT samples. Mechanical properties (σ0.2 – yield strength; σu – ultimate strength; δ – elongation to fracture; \({I_{\rm{H}}} = \left( {\frac{{{\delta _0} - {\delta _{\rm{H}}}}}{{{\delta _0}}}} \right) \cdot 100{\rm{ }}\% ;\) δ0 and δH – elongation to fracture of unhydrogenated and hydrogenated samples) are summarized in Table.
Effect of hydrogen-charging on mechanical properties
|
Examination of the provided experimental data demonstrates that post-production heat treatment yields an increase in elongation to failure and a decrease in yield strength for the examined steel. This phenomenon can be attributed to a decrease in the volume fraction of δ-ferrite, as δ-ferrite possesses higher strength compared to austenite [19]. Additionally, there is a reduction in the density of interphase boundaries (austenite – δ-ferrite), which function as barriers to the movement of dislocations during deformation [6].
The process of hydrogen charging induces alterations in the mechanical properties of EBAM steel (outlined in Table). Irrespective of the ferrite content, the yield strength (σ0.2) of hydrogen-charged samples surpasses that of the original samples (deformed without hydrogen charging). This experimental observation signifies solid-solution hardening of the austenitic phase due to the presence of hydrogen atoms [20]. Notably, it’s noteworthy that in AM-ASS + HT samples, the hydrogen-induced enhancement in yield strength (\(\Delta \sigma _{0.2}^{\rm{H}}\) = 73 MPa) exceeds that in AM-ASS samples (\(\Delta \sigma _{0.2}^{\rm{H}}\) = 55 MPa), even though, as per the results of TDS analysis, the hydrogen concentration in the latter is higher (Fig. 2, a). The presence of a limited volume fraction of ferrite and interfacial boundaries, which function as traps for hydrogen atoms [21], can lead to a heightened accumulation of hydrogen within the austenite grain bodies located near the saturable surface of AM-ASS + HT samples. This accumulation contributes to their solid solution hardening. On the contrary, hydrogen transport is more restricted in AM-ASS + HT samples due to the altered morphology and reduced volume fraction of ferrite, which leads to the suppression of hydrogen transport over long distances along ferrite dendrites and a decrease in the prevalence of interfacial boundaries. Consequently, AM-ASS samples amass a greater total hydrogen concentration. In these samples, the concentration gradient across depth is evidently smaller, subsequently yielding a lower level of solid solution strengthening in the austenite phase compared to AM-ASS + HT samples where hydrogen transport is curtailed. In addition to solid solution hardening, the hydrogen concentration gradient throughout the depth is intrinsically linked to the stress gradient within the tested samples – stemming from their heterogeneous hydrogen charging. This aspect becomes more pronounced in AM-ASS + HT samples where the transfer of hydrogen atoms during charging and subsequent deformation is hindered due to the alterations in morphology and the reduction in the volume fraction of ferrite [22; 23]. Therefore, even with a lower hydrogen adsorption concentration during hydrogen charging, the yield strength of AM-ASS + HT samples, wherein hydrogen transport is limited, displays increased susceptibility to hydrogen charging: the hydrogen-induced rise in yield strength is more significant in AM-ASS + HT samples compared to AM-ASS samples. However, the hydrogen embrittlement factor IH , which quantifies the reduction in elongation to failure due to hydrogenation, is greater for AM-ASS samples (Table).
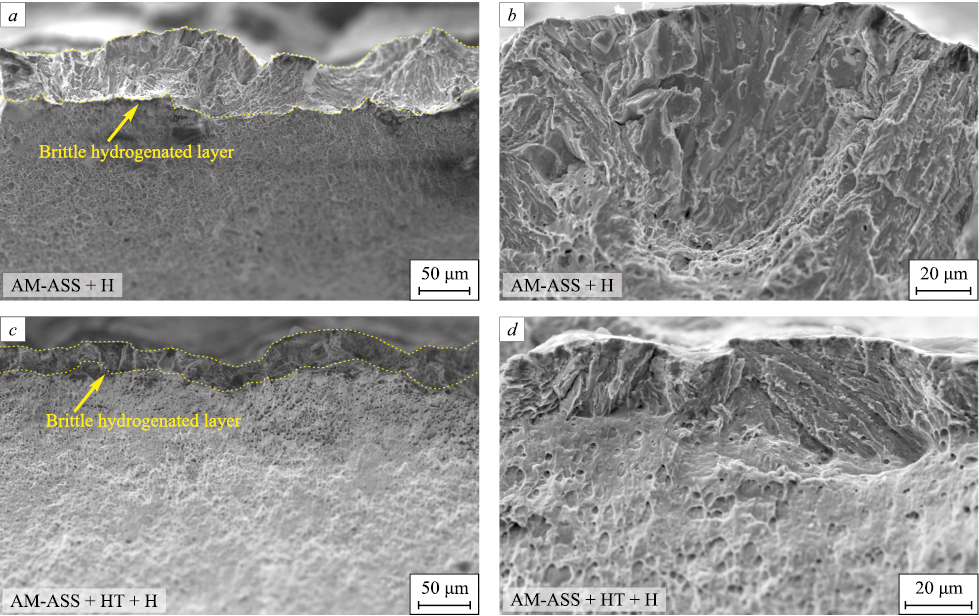
Fig. 3 portrays SEM images of fractured surfaces from both hydrogen-charged AM-ASS and AM-ASS + HT samples. Across all samples, hydrogen charging results in the formation of a brittle surface layer, while the remaining portions of the samples exhibit a ductile transcrystalline fracture mode, comparable to uncharged samples [14].
Fig. 3. SEM images of fracture surfaces of hydrogen-charged (H) AM-ASS (a, b) |
The fracture surfaces of the brittle hydrogenated layer exhibit features characteristic of both transcrystalline and intercrystalline fracture, including ridges and flat facets. The presence of intercrystalline cleavages aligns with the aforementioned mechanisms of hydrogen adsorption, specifically pointing towards the accumulation of hydrogen atoms at interphase boundaries. The transcrystalline nature of the fracture signifies the brittle fracture of hydrogen-saturated austenite grains, along with the formation of deformation-induced martensite within them, as documented in [2; 14; 23; 24].
The thickness of the brittle hydrogenated layer is notably greater for AM-ASS samples (DH = 55 ± 12 μm) characterized by a higher initial proportion of ferrite – than for AM-ASS + HT samples (DH = 29 ± 7 μm). This experimental observation corroborates the findings from TDS studies, mechanical tests, and the preceding discussions.
Conclusions
The investigation delved into the hydrogen embrittlement characteristics of austenitic chromium-nickel steel samples produced via electron beam additive manufacturing in two distinct states: immediately following additive growth and subsequent post-production heat treatment. Annealing the additively manufactured samples at 1100 °C for 1 h yielded a notable reduction in the volume fraction of δ-ferrite – from 20 % to 5 % – accompanied by an alteration in its morphology. Specifically, the state after EBAM exhibited thin extended lamellae of dendrites, while the heat-treated samples showcased isolated inclusions (particles) of ferrite. This transformation in phase composition and microstructure exerted an impact on the steel’s susceptibility to hydrogen embrittlement, the solubility and distribution of hydrogen during the process of electrolytic saturation, and the dimensions of the brittle hydrogenated layer within the samples.
Even though the annealed samples with a lower proportion of ferrite contained a lower overall concentration of dissolved hydrogen, their hydrogen-induced increase in yield strength (\(\Delta \sigma _{0.2}^{\rm{H}}\) = 73 MPa), exceeded that of samples following EBAM, characterized by a higher proportion of dendritic ferrite (\(\Delta \sigma _{0.2}^{\rm{H}}\) = 55 MPa). This discrepancy can be attributed to the impedance of hydrogen transport deep into the samples along δ-ferrite dendrites due to modifications in their morphology and the reduction of interphase boundaries. These boundaries function as traps for hydrogen atoms within both the austenite crystal lattice and intergranular regions. Consequently, post heat treatment, hydrogen transport deep into the samples is curtailed, leading to its accumulation in the surface layers. This accumulation contributes to robust solid-solution hardening of the austenitic phase, thereby engendering a pronounced stress gradient due to the hydrogen concentration gradient in these sample.
The thickness of the brittle hydrogenated surface layer and the hydrogen embrittlement factor were found to be more significant for the initial additively manufactured steel samples (DH = 55 ± 12 μm, IH = 32 % for AM-ASS and DH = 29 ± 7 μm, IH = 24 % for AM-ASS + HT samples). The reduction in the volume fraction and the alteration in the morphology of δ-ferrite, brought about by post-production heat treatment, enhance the resistance of EBAM stainless steel to hydrogen embrittlement.
References
1. Michler T., Naumann J. Hydrogen embrittlement of Cr–Mn–N-austenitic stainless steels. International Journal of Hydrogen Energy. 2010;35(3):1485–1492. https://doi.org/10.1016/j.ijhydene.2009.10.050
2. Xu X., An J., Wen C., Niu J. Study on the hydrogen embrittlement susceptibility of AISI 321 stainless steel. Engineering Failure Analysis. 2021;122:105212. https://doi.org/10.1016/j.engfailanal.2020.105212
3. Bajaj P., Hariharan A., Kini A., Kurnsteiner P., Raabe D., Jagle E.A. Steels in additive manufacturing: A review of their microstructure and properties. Materials Science and Engineering: A. 2020;772:138633. https://doi.org/10.1016/j.msea.2019.138633
4. Li N., Huang S., Zhang G., Qin R.Y., Liu W., Xiong H., Shi G., Blackburn J. Progress in additive manufacturing on new materials: A review. Journal of Materials Science & Technology. 2019;35(2):242–269. https://doi.org/10.1016/j.jmst.2018.09.002
5. Tarasov S.Yu., Filippov A.V., Shamarin N.N., Fortuna S.V., Maier G.G., Kolubaev E.A. Microstructural evolution and chemical corrosion of electron beam wire-feed additively manufactured AISI 304 stainless steel. Journal of Alloys and Compounds. 2019;803:364–370. https://doi.org/10.1016/j.jallcom.2019.06.246
6. Astafurova E.G., Panchenko M.Yu., Moskvina V.A., Maier G.G., Astafurov S.V., Melnikov E.V., Fortuna A.S., Reunova K.A., Rubtsov V.E., Kolubaev E.A. Microstructure and grain growth inhomogeneity in austenitic steel produced by wire-feed electron beam melting: the effect of post-building solid-solution treatment. Journal of Materials Science. 2020;55:9211–9224. https://doi.org/10.1007/s10853-020-04424-w
7. Chen X., Li J., Cheng X., He B., Wang H., Huang Zh. Microstructure and mechanical properties of the austenitic stainless steel 316L fabricated by gas metal arc additive manufacturing. Materials Science and Engineering: A. 2017;703: 567–677. https://doi.org/10.1016/j.msea.2017.05.024
8. Kolubaev E.A., Rubtsov V.E., Chumaevskii A.V., Astafurova E.G. Scientific approaches to micro-, meso- and macrostructural design of bulk metallic and polymetallic materials by wire-feed electron-beam additive manufacturing. Fizicheskaya mezomekhanika. 2022;25(4):5–18. (In Russ.). https://doi.org/10.55652/1683-805X_2022_25_4_5
9. Astafurov S., Astafurova E. Phase composition of austenitic stainless steels in additive manufacturing: A review. Metals. 2021;11(7):1052. https://doi.org/10.3390/met11071052
10. Baek S.W., Song E.J., Kim J.H., Jung M., Baek U.B., Nahm S.H. Hydrogen embrittlement of 3-D printing manufactured austenitic stainless steel part for hydrogen service. Scripta Materialia. 2017;130:87–90. https://doi.org/10.1016/j.scriptamat.2016.11.020
11. Li S., Liu M., Ren Y., Wang Y. Hydrogen embrittlement behaviors of additive manufactured maraging steel investigated by in situ high-energy X-ray diffraction. Materials Science and Engineering: A. 2019;766:138341. https://doi.org/10.1016/j.msea.2019.138341
12. Alnajjar M., Christien F., Bosch C., Wolski K. A comparative study of microstructure and hydrogen embrittlement of selective laser melted and wrought 17–4PH stainless steel. Materials Science & Engineering: A. 2020;785:139363. https://doi.org/10.1016/j.msea.2020.139363
13. Panchenko M.Yu., Mel’nikov E.V., Astafurov S.V., Reunova K.A., Kolubaev E.A., Astafurova E.G. Features of hydrogen embrittlement of low-carbon steel obtained by electron beam additive manufacturing. Izvestiya vuzov. Fizika. 2022;65(6):53–60. (In Russ.). https://doi.org/10.17223/00213411/65/6/53
14. Yin Q., Chen G., Cao H., Zhang G., Zhang B., Wei S. Transformation law of microstructure evolution and mechanical properties of electron beam freeform fabricated 321 austenitic stainless steel. Vacuum. 2021;194:110594. https://doi.org/10.1016/j.vacuum.2021.110594
15. Depover T., Verbeken K. The effect of TiC on the hydrogen induced ductility loss and trapping behavior of Fe–C–Ti alloys. Corrosion Science. 2016;112:308–326. https://doi.org/10.1016/j.corsci.2016.07.013
16. Escobar P.D., Depover T., Duprez L., Verbeken K., Verhaege M. Combined thermal desorption spectroscopy, differential scanning calorimetry, scanning electron microscopy and X-ray diffraction study of hydrogen trapping in cold deformed TRIP steel. Acta Materialia. 2012;60(6-7): 2593–2605. https://doi.org/10.1016/j.actamat.2012.01.026
17. Claeys L., Depover T., De Graeve I., Verbeken K. Electrochemical hydrogen charging of duplex stainless steel. Corrosion. 2019;75(8):880–887. https://doi.org/10.5006/2959
18. Owczarek E., Zakroczymski T. Hydrogen transport in a duplex stainless steel. Acta Materialia. 2000;48(12): 3059–3070. https://doi.org/10.1016/S1359-6454(00)00122-1
19. Abraham D.P., Altstetter C.J. The effect of hydrogen on the yield and flow stress of an austenitic stainless steel. Metallurgical and Materials Transactions A. 1995;26:2849–2858. https://doi.org/10.1007/BF02669643
20. Alvarez-Armas I., Degallaix-Moreuil S. Duplex Stainless Steels. Wiley-ISTE; 2009:464.
21. Panchenko M.Yu., Melnikov E.V., Mikhno A.S., Maier G.G., Astafurov S.V., Moskvina V.A., Reunova K.A., Galchenko N.K., Astafurova E.G. The influence of intergranular and interphase boundaries and δ-ferrite volume fraction on hydrogen embrittlement of high-nitrogen steel. International Journal of Hydrogen Energy. 2021;46(59):30510–30522. https://doi.org/10.1016/j.ijhydene.2021.06.183
22. Brass A.M., Chêne J. Hydrogen uptake in 316L stainless steel: Consequences on the tensile properties. Corrosion Science. 2006;48(10):3222–3242. https://doi.org/10.1016/j.corsci.2005.11.004
23. Hahnenberger F., Smaga M., Eifler D. Influence of γ–α’-phase transformation in metastable austenitic steels on the mechanical behavior during tensile and fatigue loading at ambient and lower temperatures. Advanced Engineering Materials. 2012;14(10):853–858. https://doi.org/10.1002/adem.201100341
24. Koyama M., Tasan C.C., Tsuzaki K. Overview of metastability and compositional complexity effects for hydrogen-resistant iron alloys: Inverse austenite stability effects. Engineering Fracture Mechanics. 2019;214:123–133. https://doi.org/10.1016/j.engfracmech.2019.03.049
25.
About the Authors
M. Yu. PanchenkoRussian Federation
Marina Yu. Panchenko, Junior Researcher of the Laboratory of Physics of Hierarchical Structures in Metals and Alloys
2/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
K. A. Reunova
Russian Federation
Kseniya A. Reunova, Postgraduate, Junior Researcher of the Laboratory of Physics of Hierarchical Structures in Metals and Alloys
2/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
A. S. Nifontov
Russian Federation
Aleksei S. Nifontov, Research Laboratory Assistant of the Laboratory of Physics of Hierarchical Structures in Metals and Alloys
2/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
E. A. Kolubaev
Russian Federation
Evgenii A. Kolubaev, Dr. Sci. (Eng.), Director
2/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
E. G. Astafurova
Russian Federation
Elena G. Astafurova, Dr. Sci. (Phys.-Math.), Assist. Prof., Head of the Laboratory of Physics of Hierarchical Structures in Metals and Alloys
2/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
Review
For citations:
Panchenko M.Yu., Reunova K.A., Nifontov A.S., Kolubaev E.A., Astafurova E.G. Effect of morphology and volume fraction of δ-ferrite on hydrogen embrittlement of stainless steel produced by electron beam additive manufacturing. Izvestiya. Ferrous Metallurgy. 2023;66(4):434-441. https://doi.org/10.17073/0368-0797-2023-4-434-441
JATS XML