Scroll to:
Grain structure formation and microhardness of Ni\(_{3}\)Al intermetallic compound fabricated by SHS extrusion
https://doi.org/10.17073/0368-0797-2023-1-57-61
Abstract
In this paper we studied the possibility to enhance the microhardness of Ni3Al intermetallic compound by reducing the average grain size and the effect of the mixture deformation during self-propagating high-temperature synthesis (SHS) on the Ni3Al grain size and microhardness. We used an SHS extrusion test bench to continuously monitor the synthesis variables. One of the key factors affecting the grain structure and microhardness is deformation rate of the synthesis product. Increasing the extrusion nozzle diameter from 3 to 5 mm results in a longer displacement of the press plunger since it takes less force to extrude the material through the larger diameter orifice. It is assumed that the resistance to deformation under pressure decreases, while the deformation rate increases for the material in the mold, and decreases for the extruded material. As a result, the average grain size of Ni3Al remaining in the mold after synthesis decreases by 40 % (from 7 to 5 μm), while the grain size of the extruded material is doubled (from 3 to 6 μm). Compared to Ni3Al produced by SHS compaction, the average grain size of extruded Ni3Al is 82 % less (17 and 3 μm, respectively). Reducing the average grain size of extruded Ni3Al leads to a 600 MPa increase in microhardness. The results obtained may assist the development of guidelines for fine grain, high microhardness intermetallide/alloy manufacturing.
For citations:
Akimov K.O., Ivanov K.V., Figurko M.G., Ovcharenko V.E. Grain structure formation and microhardness of Ni\(_{3}\)Al intermetallic compound fabricated by SHS extrusion. Izvestiya. Ferrous Metallurgy. 2023;66(1):57-67. https://doi.org/10.17073/0368-0797-2023-1-57-61
Introduction
The Ni3Al intermetallic compound is the key component of advanced nickel-based superalloys providing high strength at elevated temperatures, and creep resistance [1 – 4]. Despite the excellent mechanical properties at high temperatures, the applications of Ni3Al are limited. This is due to its low ductility at room temperature caused by intergranular embrittlement which significantly complicates any machining [5 – 8]. As was shown in [9, 10], the ductility and strength of Ni3Al can be increased by alloying or refining the grains, for example, by severe plastic deformation [11 – 14]. Still, severe plastic deformation is suitable for small samples only such as disks ~0.5 mm thick and up to 15 mm in diameter under torsion and pressure [15], or plates less than 30 μm thick under multi-pass rolling [16].
Grain refinement in large Ni3Al samples requires deformation at temperatures close to the melting point. Such conditions are achievable as the workpiece is crystallized during the self-propagating high-temperature synthesis and partial extrusion (SHS extrusion) [17, 18]. The phase transformations occur simultaneously in the bulk of pressed powder [19] during the volumetric exothermic reaction of Ni3Al synthesis from the powder mixture of nickel and aluminum. Deformation of the mixture is a way to control the average grain size of the Ni3Al compound synthesized under pressure [20, 21].
The purpose of this study was to investigate the effect of mixture deformation during SHS extrusion on the average grain size and microhardness of the Ni3Al compound.
Materials and Methods
We used a test hydraulic press for the SHS extrusion of Ni3Al. The press was equipped with an HF generator for inductive mold heating [22]. We processed a mixture of nickel (PNK-1L8, particle size: 1 – 5 μm) and aluminum (ASD-4, particle size: 1 – 4 μm) powders. The powder mixture was placed in a steel mold, 58 mm inner diameter, 3 to 5 mm extrusion nozzle diameter. The process temperature (inside the steel mold wall) was measured with a type K thermocouple, with ±7 °C accuracy. The pressure was calculated from the press instrument readings pressure and the punch area. The press plunger displacement was continuously measured using a Shahe 5403-200 digital linear scale, of ±0.6 mm accuracy.
The high-temperature synthesis of the Ni3Al intermetallic compound from a mixture of nickel and aluminum powders under pressure consists of several steps: preloading the powder mixture (3Ni + Al) in the mold (at 115 MPa); heating the pressed powder until (Ni-Al)-eutectics is formed; melting the aluminum component; initiation of the exothermic Ni3Al formation simultaneously with applying the specified pressure to the high-temperature synthesis product; holding the final product at the specified pressure (430 MPa).
The key process variables which control the grain structure of the synthesized intermetallide are the deformation rate of the mixture in the mold and the period of pressure applied to the thermally reacting powder mixture after the intermetallide synthesis reaction is initiated.
We manufactured Ni3Al cylinders with concave end faces, 58 mm diameter, 16 mm height in the middle by high-temperature synthesis under pressure at different deformation rates, and partial extrusion of the synthesis product from the mold through 3, 4, and 5 mm dia. nozzles. The pressure was applied 1 s after the reaction initiation. The holding time is 1 s, in order to ensure uniform pressure distribution across the mold which has extrusion nozzles of different diameters. An increase in the extrusion nozzle diameter leads to an increase in the mixture deformation rate in the mold. After passing through the extrusion nozzle, the material was shaped as a rod up to 180 mm long.
The samples of the material remaining in the mold were cut from the middle vertical cross sections as 1 mm thick plates, in order to eliminate the edge effects which may change the structure and properties of the material. The samples of the extruded material were cut from a cross-section passing through the axial axis. The samples were mechanically ground with a diamond paste. The abrasive particle size was gradually reduced to 1 μm. Then the samples were polished with an aluminum oxide suspension (0.3 μm particle size) and cloth. In order to reveal the grain structure, we applied argon ion etching, 0.6 kV accelerating voltage. A Carl Zeiss AXIOVERT-200MAT microscope was used for metallographic analysis. The average grain size was measured by the random plane method according to GOST 5639-82 (150 measurements). We measured the microhardness with a PMT-3 microhardness meter. The indenter load was 0.98 N, and the loading time was 15 s. The final microhardness was the mean value of at least 10 tests.
Results and Discussion
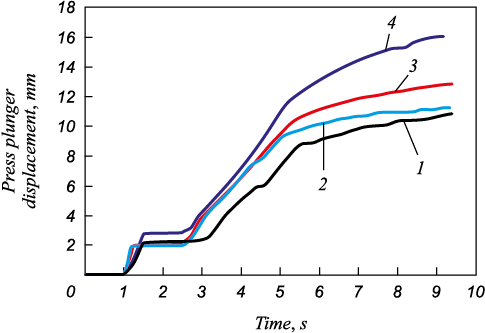
Fig. 1 shows the press plunger position vs. time curves at the mixture compression stage during the SHS extrusion through extrusion 3, 4, and 5 mm dia. nozzles.
Fig. 1. Dependences of linear movement of press |
The plunger displacement in the mold increases with the diameter of the extrusion nozzle. This indicates a decrease in the resistance to deformation under pressure. It is assumed that the deformation rate of the synthesis product within the mold increases and that of the extruded material decreases.
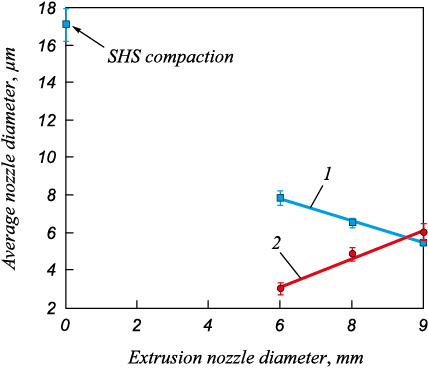
Fig. 2 shows that using larger nozzle diameters leads to a 40 % decrease in the average Ni3Al grain size of the material remaining in the mold after synthesis (from 7 ± 0.4 to 5 ± 0.3 μm). With regard to the extruded material, reducing the nozzle diameter from 5 to 3 mm results in halving the average grain size (from 6 ± 0.5 to 3 ± 0.3 µm). Compared to the Ni3Al compound made by SHS compaction, the average grain size is 82 % less (17 ± 0.5 and 3 ± 0.3 μm). SHS extrusion reduces the average grain size due to the higher deformation rate as the material exits the mold into the extrusion channel with a smaller nozzle diameter.
Fig. 2. Dependence of the average grain size of Ni3Al |
It should be noted that the Ni3Al average grain size vs. extrusion nozzle diameter curve confirms the above assumption that, as the nozzle diameter increases, the deformation rate of the synthesis product remaining in the mold increases and that of the extruded material decreases.
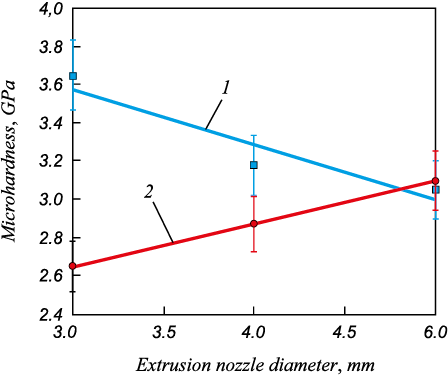
Fig. 3 shows the hardness curves for Ni3Al made by SHS extrusion. It can be seen that changing the extrusion nozzle diameter resulting in a decrease in the average Ni3Al grain size leads to an increase in the intermetallide microhardness by 16, and 20 % for the material remaining inside the mold after synthesis and the extruded material, respectively.
Fig. 3. Dependences of microhardness of Ni3Al intermetallic |
The results are consistent with the data reported by Stolin A.M., etc. [23]. They indicate that SHS extrusion is suitable for making long products from brittle, hard-to-deform refractory materials such as Ni3Al.
Conclusions
Deformation of the high-temperature synthesis product during SHS extrusion significantly affects the average grain size and microhardness of the Ni3Al intermetallide (both remaining in the mold and extruded). The higher deformation rate of the material inside the mold as the extrusion nozzle diameter is increased from 3 to 5 mm, leads to a 30 % decrease in the average grain size and a 16 % increase in the microhardness. For the extruded material, as the extrusion nozzle diameter is reduced from 5 to 3 mm, the average grain size is halved, and the microhardness is increased by 20 % (600 MPa).
Our findings can be used to develop fine-grade, high-microhardness intermetallide/alloy SHS extrusion processes.
References
1. Liu C.T., Sikka V.K. Nickel aluminides for structural use. Journal of Mechanical Engineering and Technology. 1986; 38: 19–21. https://doi.org/10.1007/BF03257837
2. Taub A.I., Fleischer R.L. Intermetallic compounds for high-temperature structural use. New Series. 1989; 243(4891): 616–621. https://doi.org/10.1126/science.243.4891.616
3. Amrit R.P., Manidipto M., Dilpreet S. A critical review on the properties of intermetallic compounds and their application in the modern manufacturing. Crystal Research and Technology. 2022; 57(3): 2100159. https://doi.org/10.1002/crat.202100159
4. Tewari R., Sarkar N.K., Harish D., Vishwanadh B., Dey G.K., Banerjee S. Intermetallics and alloys for high temperature applications materials under extreme conditions. In: Materials Under Extreme Conditions. Tyagi A.K. ed. Amsterdam: Elsevier; 2017: 293–335. https://doi.org/10.1016/B978-0-12-801300-7.00009-7
5. Stoloff N.S., Liu C.T., Deevi S.C. Emerging application of intermetallics. Intermetallics. 2008; 8(9-11): 1313–1320. https://doi.org/10.1016/S0966-9795(00)00077-7
6. Sikka V.K., Deevi S.C., Viswanathan S., Swindeman R.W., Santella M.L. Advances in processing of Ni3Al -based intermetallics and applications. Intermetallics. 2000; 8(9-11): 1329–1337. https://doi.org/10.1016/S0966-9795(00)00078-9
7. Pope D.P., Ezz S.S. Mechanical properties of Ni3Al and nickel-base alloys with high volume fraction of γ′. International Materials Reviews. 1984; 29(1): 136–167. https://doi.org/10.1179/imtr.1984.29.1.136
8. Deevi S.C., Sikka V.K. Nickel and iron aluminides: an overview on properties, processing and applications. Intermetallics. 1996; 4(5): 357–375. https://doi.org/10.1016/0966-9795(95)00056-9
9. Schulson E.M., Baker I., Frost H.J. The strength and ductility of intermetallic compounds: Grain size effects. Materials Research Society Symposia Proceedings. 1986; 81: 195–205. https://doi.org/10.1557/PROC-81-195
10. Polkowski W., Jóźwik P., Karczewski K., Bojar Z. Evolution of crystallographic texture and strain in a fine-grained Ni3Al (Zr, B) intermetallic alloy during cold rolling. Archives of Civil and Mechanical Engineering. 2014; 14(4): 550–560. https://doi.org/10.1016/j.acme.2014.04.011
11. Valiev R.Z., Estrin Y., Horita Z., Langdon T.G., Zechetbauer M.J., Zhu Y.T. Producing bulk ultrafine-grained materials by severe plastic deformation. JOM. 2006; 58: 33–39. https://doi.org/10.1007/s11837-006-0213-7
12. Valiev R.Z., Alexandrov I.V., Zhu Y.T., Lowe T.C. Paradox of strength and ductility in metals processed by severe plastic deformation. Journal of Materials Research. 2002; 17: 5–8. https://doi.org/10.1557/JMR.2002.0002
13. Jóźwik P., Bojar Z. Analysis of grain size effect on tensile properties of Ni3Al – based intermetallic strips. Archives of Metallurgy and Materials. 2007; 52(2): 321–327.
14. McFadden S., Mishra R., Valiev R., Zhilyaev A.P., Mukherjee A.K. Low-temperature superplasticity in nanostructured nickel and metal alloys. Nature. 1998; 398: 684–686. https://doi.org/10.1038/19486
15. Korznikov A.V., Idrisova S.R., Dimitrov O., Pyshmintsev I., Sirenko A.A., Korznikova G. Structure and mechanical properties of the nanocrystalline intermetallic compound Ni3Al. The Physics of Metals and Metallography. 1998; 85(5): 564–567.
16. Demura M., Kishida K., Suga Y., Takanashi M., Hirano T. Fabrication of thin Ni3Al foils by cold rolling. Scripta Materialia. 2022; 47(4): 267–272. https://doi.org/10.1016/S1359-6462(02)00139-2
17. Lebrat J.P., Varma A. Self-propagating high-temperature synthesis of Ni3Al. Combustion Science and Technology. 1992; 88(3-4): 211–221. https://doi.org/10.1080/00102209308947237
18. Hibino A., Matsuoka S., Kiuchi M. Synthesis and sintering of Ni3Al intermetallic compound by combustion synthesis process. Journal of Materials Processing Technology. 2001; 112(1): 127–135. https://doi.org/10.1016/S0924-0136(01)00558-1
19. Merzhanov A.G. History and recent developments in SHS. Ceramics International. 1995; 21(5): 371–379. https://doi.org/10.1016/0272-8842(95)96211-7
20. Ovcharenko V.E., Lapshin O.V., Ramazanov I.S. Formation of the granular structure in the intermetallic compound Ni3Al in high-temperature synthesis under compression. Combustion, Explosion and Shock Waves. 2006; 42(3): 302–308. https://doi.org/10.1007/s10573-006-0055-1
21. Si J., Gao F., Han P. Zhang J. Simulation on extrusion process of TiAl alloy. Intermetallics. 2011; 19(2): 169–174. https://doi.org/10.1016/j.intermet.2010.08.021
22. Ovcharenko V.E., Akimov K.O. Effect of deformation on the grain size of the Ni3Al intermetallic compound synthesized under pressure. Inorganic Materials. 2020; 56(11): 1122–1126. https://doi.org/10.1134/S0020168520110114
23. Stolin A.M., Bazhin P.M. Manufacture of multipurpose composite and ceramic materials in the combustion regime and high-temperature deformation (SHS Extrusion). Theoretical Foundations of Chemical Engineering. 2014; 48(6): 751–763. https://doi.org/10.1134/S0040579514060104
About the Authors
K. O. AkimovRussian Federation
Kirill O. Akimov, Junior Researcher of the Laboratory of Physics of Consolidation of Powder Materials
2/4 Akademicheskii Ave., Tomsk, 634055, Russian Federation
K. V. Ivanov
Russian Federation
Konstantin V. Ivanov, Dr. Sci. (Phys.-Math.), Leading Researcher of the Laboratory of Physics of Consolidation of Powder Materials
2/4 Akademicheskii Ave., Tomsk, 634055, Russian Federation
M. G. Figurko
Russian Federation
Marina G. Figurko, Engineer of the Laboratory of Physics of Consolidation of Powder Materials
2/4 Akademicheskii Ave., Tomsk, 634055, Russian Federation
V. E. Ovcharenko
Russian Federation
Vladimir E. Ovcharenko, Dr. Sci. (Eng.), Prof., Chief Researcher of the Laboratory of Composite Materials
2/4 Akademicheskii Ave., Tomsk, 634055, Russian Federation
Review
For citations:
Akimov K.O., Ivanov K.V., Figurko M.G., Ovcharenko V.E. Grain structure formation and microhardness of Ni\(_{3}\)Al intermetallic compound fabricated by SHS extrusion. Izvestiya. Ferrous Metallurgy. 2023;66(1):57-67. https://doi.org/10.17073/0368-0797-2023-1-57-61