Scroll to:
Preliminary assessment of X52 large-diameter pipes suitability for transportation of pressurized pure gaseous hydrogen
https://doi.org/10.17073/0368-0797-2023-1-35-42
Abstract
In order to assess the resistance to hydrogen embrittlement caused by the presence of hydrogen in the transported product, and, accordingly, the suitability of pipes for transporting hydrogen, we studied the metal of large-diameter X52 strength class pipes manufactured by JSC “ChelPipe” (a TMK Group company). The work included the study of pure gaseous hydrogen effect under pressure up to 10 MPa on change in mechanical characteristics of the base metal of large-diameter pipes (LDP) during preliminary hydrogen charging for various periods in a stationary autoclave under pressure, and during simultaneous loading with a slow strain rate (SSRT) under expected operating conditions. Results of the X52 LDP metal study show that there is no significant impact on the effect of gaseous hydrogen under pressure for up to 144 hours on mechanical characteristics of the base metal determined by static uniaxial tension (decrease in ductile characteristics does not exceed 9 %). During SSRT at a rate of not more than 1·10–6 s–1 in a pure gaseous hydrogen environment under a pressure of 10 MPa, the change in strength and ductile characteristics does not exceed 13 % in comparison with the reference tests in a nitrogen environment under the same pressure. The results obtained allow us to consider that the base metal of low-alloy pipe steel with ferrite-perlite microstructure of X52 strength class is sufficiently resistant to hydrogen embrittlement. Final confirmation of the possibility of using LDP made from steel under study will be the results of further qualification tests, including the study of the weld metal and heat-affected zone properties.
Keywords
For citations:
Pyshmintsev I.Yu., Gizatullin A.B., Devyaterikova N.A., Laev K.A., Tsvetkov A.S., Alkhimenko A.A., Shaposhnikov N.O., Kurakin M.K. Preliminary assessment of X52 large-diameter pipes suitability for transportation of pressurized pure gaseous hydrogen. Izvestiya. Ferrous Metallurgy. 2023;66(1):35-42. https://doi.org/10.17073/0368-0797-2023-1-35-42
Introduction
One of the current challenges is the transition to hydrogen energy. There is extensive research to support the development of hydrogen transportation and storage infrastructure and the use of existing gas pipeline grids to deliver hydrogen. One important issue is the properties of materials which come into contact with pure gaseous hydrogen under pressure1 [1].
For this reason, the Scientific and Technological Complex «New Technologies and Materials» at St. Petersburg Peter the Great Polytechnic University initiated a project to study the changes in structure and properties of pipe steel grades after hydrogen charging in a pure gaseous hydrogen environment under pressure [2 – 5]. According to several studies [6 – 10], the advanced steel grades used in main pipelines are promising for hydrogen transportation.
The structural strength, microstructure, and other properties may vary greatly in hydrogen, air, or inert environments. As indicated by tensile tests with standard and extremely slow strain rates, the elasticity is affected the most when the fracture pattern is changed. The purpose of this study is to estimate the degradation of the large-diameter pipe (LDP) material properties. The pipes are made of ferrite-perlite plate steel when exposed to gaseous hydrogen at high pressure equal to the operating pressure in today’s long-distance gas transportation grids.
Material Properties
We studied the samples of LDP (diameter: 1420 mm; wall thickness: 14 mm) 17G1S-U low-alloy tube steel grade (GOST 19281, X52 strength class). The EU analog is Fe52CFN. The chemical composition (as indicated in the quality certificate) in % is as follows:
| С | Mn | Si | Cr | Cu | Ni | P | S |
| 0.110 | 1.460 | 0.490 | 0.040 | 0.040 | 0.010 | 0.010 | 0.001 |
| V | Nb | Al | Ti | Nb + V + Ti | S + P | ||
| 0.006 | 0.032 | 0.031 | 0.017 | 0.055 | 0.011 | ||
Metallographic analysis showed that the steel has a ferrite-perlite structure. The average grain diameter is 7.61 µm (Fig. 1).
Fig. 1. Microstructure of base metal |
The pipe was manufactured from hot-rolled plates after controlled rolling. The pipe is made by cold gradual forming of rolled plates with a press. The pipe has one longitudinal double-sided (outer and inner) weld made by the automated flux-cored arc welding process. The pipe quality is compliant with the pipe specifications.
Test Methods
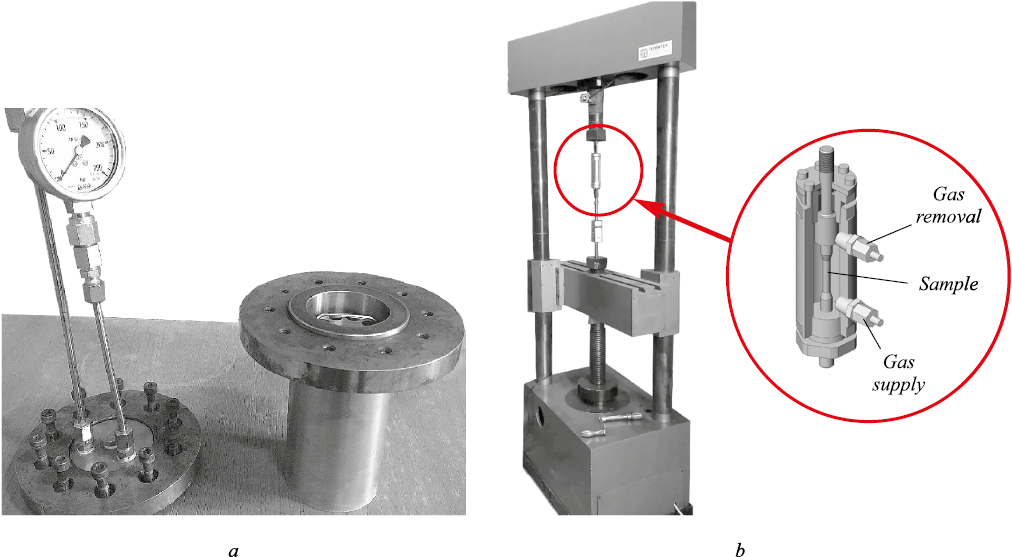
In order to evaluate the changes in the mechanical properties after preliminary hydrogen charging, the samples were kept for 72 and 144 h in pure hydrogen gas in in a stationary autoclave with a working part volume of 0.5 liters (Fig. 2, a).
Before the test, the autoclave was repeatedly purged with helium and then with hydrogen. After the pure gaseous hydrogen pressure reached 10 MPa, the samples were held for the required period. Then for 10 – 15 min, they were tested for uniaxial tension with an Instron testing machine.
The smooth cylindrical samples 6 mm dia. were tested for static uniaxial tension according to GOST 1497 and ASTM G142. The samples were cut along the main direction of strain. Two samples for each holding time were used. We compared the tested mechanical properties with that of the reference samples.
The slow strain rate testing (SSRT) complied with ASTM G129 and NACE TM0198 using a UME-10T tensile testing machine: a customized autoclave that can apply loads to the sample in a gaseous environment pressure (Fig. 2, b). The tests were performed in pure gaseous hydrogen and nitrogen (for the reference samples) at a slow strain rate of 8.5∙10–7 s–1. It complies with the NACE TM0198 requirement that the strain rate should be less or equal to 1∙10–6 s–1. The samples were smooth cylinders 6.35 mm dia. cut in the longitudinal direction. Two samples were used for each environment. After placing the samples, the autoclave was repeatedly purged with helium and then with hydrogen (for the hydrogen environment tests) or nitrogen (for the reference sample tests). The gas pressure was raised to the required value (10 MPa), and the strain was applied at the specified rate.
Fig. 2. Autoclaves used: stationary autoclave with the possibility to control and regulate pressure (a); |
We evaluated the tensile test results (also for the SSRT) by analyzing the average values of the measured structural strength and elasticity. The property changes were expressed as a percentage [6 – 8]. The strength and elasticity ratios are the ratios of the property values in a pure hydrogen environment to the property values of the reference samples.
The changes in the strength and elasticity ratios were expressed as the decrease in value relative to 100 %. When a value is close to 100 %, we may claim that the respective material property is not affected by hydrogen under the test conditions. The lower the strength and elasticity ratios, the greater the effect of the holding time in pure hydrogen at 10 MPa on the metal’s mechanical properties.
We examined the fracture surface of the samples using a Tescan MIRA3 scanning electron microscope.
Test Results
The static uniaxial tension tests of the hydrogen charged samples show a weak effect of the hydrogen charging conditions used on the elasticity properties (Fig. 3, a). The decrease in the plasticity ratios does not exceed 9 %. No significant degradation of the structural strength after such tests was found (the strength ratios are about 100 %). According to Tröger M. et al. [7], elasticity ratios above 80 % indicate high resistance to hydrogen embrittlement.
Fig. 3. Tensile test diagrams of the samples at a strain rate of 10–2 s–1 (а) and 10–6 s–1 (b): |
As the results show, longer exposure to gaseous hydrogen leads to a slight decrease in the elasticity properties of the metal. This has also been reported in many studies [6; 9 – 11]. Despite this trend, the key properties of the sample metal after holding it in pure hydrogen gas for 72 and 144 h changed insignificantly relative to the reference samples.
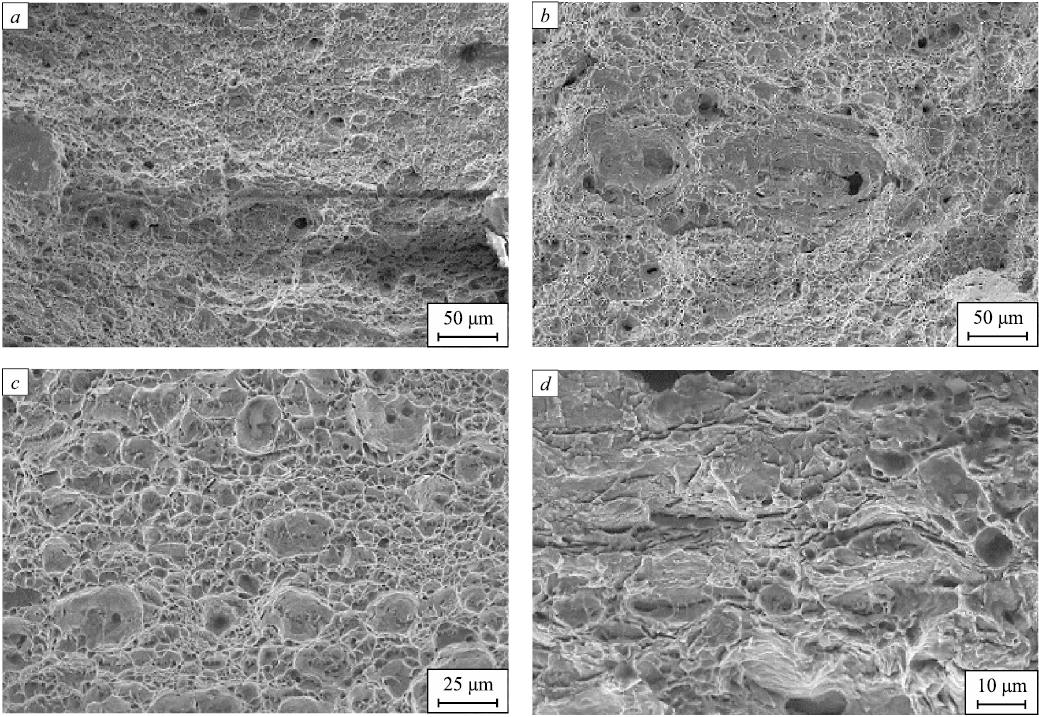
The fracture surfaces of the samples have pits. The surface pattern does not change significantly after hydrogen charging (Fig. 4, a, b).
All of the above indicates no significant effect of pure gaseous hydrogen at 10 MPa, a well as the high resistance (no changes in the strength and elasticity) of the studied metal during pre- hydrogen charging at up to 144 h holding time.
Fig. 4. Appearance of fracture surface of the tested samples: |
When testing the X52 pressure rating steel at slow strain rates in a hydrogen environment, there is a slight decrease in the strength and elasticity compared to the reference tests in a nitrogen environment. The changes in strength and elasticity of the samples tested in a hydrogen environment relative to that of the reference samples in a nitrogen environment under identical conditions do not exceed 13 %. The strength and elasticity ratios are above 80 %, which indicates the resistance of the investigated steel to loads applied in a hydrogen environment [7].
The results are consistent with other SSRT studies of hydrogen embrittlement in pipe steels in a hydrogen environment [6; 12; 13]. The tensile curves for the tested samples are shown in Fig. 3, b.
The SSRT samples tested in a nitrogen environment showed a viscous fracture pattern. The fracture surface has pits (Fig. 4, c). The slow strain rate tests in pure gaseous hydrogen produced microcracks and brittle fracture areas (Fig. 4, d).The results we obtained are similar to the fractography results presented in [11; 14; 15]. The fracturing is attributed to the accumulation of hydrogen in defects both in the surface layer of the metal and below. This can lead to high internal stress at the hydrogen concentration areas and the formation of micro- and macro-cracks. Hydrogen embrittlement requires the continuous diffusion of hydrogen from inside the metal to its surface. Thus any factors which contribute to the increase of the hydrogen volume diffusing to the crack intensify hydrogen embrittlement [16]. With a significantly lower strain rate, hydrogen has enough time to diffuse into the sample material and redistribute at the critical points of the microstructure (e.g., at the tops of cracks formed during testing) [8]. It facilitates the formation of embrittlement areas and leads to small cracks during testing (Fig. 4, d).
A decrease in the strain rate from ~10–2 s–1 (for the hydrogen charged sample testing according to GOST 1497) to ~10–6 s–1 (for SSRT in hydrogen) leads to a more noticeable, but not critical change in the plasticity ratios from 95 to 85 % on average. A similar decrease in elasticity with virtually unchanged strength was found in gaseous hydrogen SSRT tests of X80 pressure rating pipe steel [6; 11], while the loss of elasticity increases with decreasing the strain rate.
Despite the changes observed in the mechanical properties when we tested the samples after preliminary hydrogen charging and by loading in a gaseous hydrogen environment, the properties of X52 pipe steel remain within the specifications for pipes. They are consistent with the test results presented in [6 – 15] where metal embrittlement in pure hydrogen gas environment under pressure is evaluated.
Conclusions
We studied the resistance of typical low-alloy pipe steel, moderate X52 strength class rating to hydrogen embrittlement. We found no significant changes in strength and elasticity after exposure to gaseous hydrogen at 10 MPa and room temperature for 24 – 144 h. The reduction of elasticity in the samples does not exceed 10 %.
The SSRT tests (strain rate not exceeding 1∙10–6 s–1) showed a decrease in elasticity not exceeding 20 %. The greater loss of elasticity compared to the tests of pre-hydrogen charged samples is caused by the possible diffusion of hydrogen near the stress concentrators and the tops of cracks with the strain rate decrease.
Regardless of the test conditions, the key structural strength properties of the metal remain within the specifications.
Therefore, the tests (under the above conditions) indicate that the ferrite-perlite pipe steel, X52 strength class, shows good resistance to hydrogen embrittlement. Our results are in good agreement with the published results of similar tests of low-alloy plate and pipe steel grades.
The final confirmation of the X52 strength class pipe steel suitability for operations in gaseous hydrogen at pressures up to 10 MPa will be the qualification tests pursuant to ASME B 31.12 2 [17; 18] and ASME BPVC [19], as well as studies of the hydrogen effects on the weld metal and heat affected zone.
References
1. Chugunov A.V. Bebeshko I.G., Semenov A.M., Becker W., Fenin C., Hoecher T. Experimental research in the influence of a mixture of methane and oxygen gases uponstructural and mechanical properties of several steel grades. Gazovaya promyshlennost’. 2016; 10: 82–89. (In Russ.).
2. Kolesov S., Alekseeva E. Evaluation of the stress-strain state in alloy 718 after hydrogen charging. In: Proceedings of the 9th Int. Symp. on Superalloy 718 & Derivatives: Energy, Aerospace, and Industrial Applications. The Minerals, Metals & Materials Series. 2018: 553–563. https://doi.org/10.1007/978-3-319-89480-5_36
3. Kolesov S., Badrak R., Shakhmatov A. Hydrogen influence on crack propagation and stress-strain evolution of alloy 718. In: Proceedings of the 9th Int. Symp. on Superalloy 718 & Derivatives: Energy, Aerospace, and Industrial Applications. The Minerals, Metals & Materials Series. 2018: 209–218. https://doi.org/10.1007/978-3-319-89480-5_12
4. Frolova K., Vilchevskaya E., Polyanskiy V., Alekseeva E. Modelling of a hydrogen saturated layer within the micropolar approach. New Achievements in Continuum Mechanics and Thermodynamics. 2019; 108: 117–128. https://doi.org/10.1007/978-3-030-13307-8_9
5. Polyanskiy V.A., Belyaev A.K., Alekseeva E.L., Tretyakov D.A., Yakovlev Y.A. Phenomenon of skin effect in metals due to hydrogen absorption. Continuum Mechanics and Thermodynamics. 2019; 31: 1961–1975. https://doi.org/10.1007/s00161-019-00839-2
6. Nanninga N.E., Levy Y.S., Drexler E.S., Condon R.T., Stevenson A.E., Slifka A.J. Comparison of hydrogen embrittlement in three pipeline steels in high pressure gaseous hydrogen environments. Corrosion Science. 2012; 59: 1–9. https://doi.org/10.1016/j.corsci.2012.01.028
7. Tröger M., Bosch C., Wiart J.-N., Meuser H., Knoop F.M., Brauer H., Schröder J. Investigations on hydrogen assisted cracking of welded high-strength pipes in gaseous hydrogen. Steely Hydrogen Conf. Proceedings – 2014. 2014: 491–501.
8. Brauer H., Simm M., Wanzenberg E., Henel M., Huising O.J. Energy transition with hydrogen pipes: Mannesmann “H2ready” and the changeover. Pipeline Technology. 2020; 1: 16–29.
9. Meng B., Gu C., Zhang L., Zhou C., Li X., Zhao Y., Zheng J., Chen X., Han Y. Hydrogen effects on X80 pipeline steel in high-pressure natural gas/hydrogen mixtures. International Journal of Hydrogen Energy. 2017; 42(11): 7404–7412. https://doi.org/10.1016/J.IJHYDENE.2016.05.145
10. Zhou D., Li T., Huang D., Wu Y., Huang Z., Xiao W., Wang Q., Wang X. The experiment study to assess the impact of hydrogen blended natural gas on the tensile properties and damage mechanism of X80 pipeline steel. International Journal of Hydrogen Energy. 2021; 46(10): 7402–7414. https://doi.org/10.1016/j.ijhydene.2020.11.267
11. Briottet L., Moro I., Lemoine P. Quantifying the hydrogen embrittlement of pipeline steels for safety considerations. International Journal of Hydrogen Energy. 2012; 37(22): 17616–17623. https://doi.org/10.1016/j.ijhydene.2012.05.143
12. Somerday B.P. Technical reference on hydrogen compatibility of materials. Plain carbon ferritic steels: C-Mn alloys (code 1100). URL: https://www.sandia.gov/app/uploads/sites/158/2021/12/1100TechRef_FeCMn.pdf (Accessed 13.01.2023).
13. Bolobov V.I., Latipov I.U., Popov G.G., Buslaev G.V., Martynenko Ya.V. Estimation of the influence of compressed hydrogen on the mechanical properties of pipeline steels. Energies. 2021; 14(19): 6085. https://doi.org/10.3390/en14196085
14. Nguyen T.T., Park J., Kim W.S., Nahm S.H., Baek U.B. Effect of low partial hydrogen in a mixture with methane on the mechanical properties of X70 pipeline steel. International Journal of Hydrogen Energy. 2020; 45(3): 2368–2381. https://doi.org/10.1016/j.ijhydene.2019.11.013
15. Joseph A., Ronevich J.A., Song E.J., Somerday B.P., San Marchi C.W. Hydrogen-assisted fracture resistance of pipeline welds in gaseous hydrogen. International Journal of Hydrogen Energy. 2021; 46(10): 7601–7614. https://doi.org/10.1016/j.ijhydene.2020.11.239
16. Kolachev B.A. Hydrogen Brittleness of Metals. Moscow: Metallurgiya; 1985: 216. (In Russ.).
17. Martin M.L., Connolly M., Buck Z.N., Bradley P.E., Lauria D., Slifka A.J. Evaluating a natural gas pipeline steel for blended hydrogen service. Journal of Natural Gas Science and Engineering. 2022; 101: 104529. https://doi.org/10.1016/j.jngse.2022.104529
18. ASME B31.12-2019. Hydrogen Piping and Pipelines. USA, ASME; 2020: 280.
19. ASME BPVC. VIII. 3 – 2017. Boiler & Pressure Vessel Code. Division 3 Alternative Rules for Construction of High Pressure Vessels. USA, ASME; 2017: 407.
About the Authors
I. Yu. PyshmintsevRussian Federation
Igor’ Yu. Pyshmintsev, Dr. Sci. (Eng.), Prof., Director for Research, JSC “TMK”, General Director, LLC “Research and Development Centre TMK”, General Director, Russian Scientific Research Institute of the Pipe Industry
40/2a Pokrovka Str., Moscow 101000, Russian Federation
A. B. Gizatullin
Russian Federation
Anton B. Gizatullin, Deputy Director for Research
40/2a Pokrovka Str., Moscow 101000, Russian Federation
N. A. Devyaterikova
Russian Federation
Natal’ya A. Devyaterikova, Chief Specialist of Industrial Pipe Center
40/2a Pokrovka Str., Moscow 101000, Russian Federation
K. A. Laev
Russian Federation
Konstantin A. Laev, Cand. Sci. (Eng.), Chief Specialist of Industrial Pipe Center
40/2a Pokrovka Str., Moscow 101000, Russian Federation
A. S. Tsvetkov
Russian Federation
Anton S. Tsvetkov, Cand. Sci. (Eng.), Engineer, Deputy Head of the Experimental Laboratory of the Scientific and Technical Complex “New Technologies and Materials” of the Center for Scientific and Technical Research
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
A. A. Alkhimenko
Russian Federation
Alexei A. Alkhimenko, Director of the Scientific and Technological Complex “New Technologies and Materials”
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
N. O. Shaposhnikov
Russian Federation
Nikita O. Shaposhnikov, Executive Director of the Scientific and Technological Complex “New Technologies and Materials”
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
M. K. Kurakin
Russian Federation
Maksim K. Kurakin, Engineer, Project Manager of the Scientific and Technical Complex “New Technologies and Materials” of the Center for Scientific and Technical Research
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
Review
For citations:
Pyshmintsev I.Yu., Gizatullin A.B., Devyaterikova N.A., Laev K.A., Tsvetkov A.S., Alkhimenko A.A., Shaposhnikov N.O., Kurakin M.K. Preliminary assessment of X52 large-diameter pipes suitability for transportation of pressurized pure gaseous hydrogen. Izvestiya. Ferrous Metallurgy. 2023;66(1):35-42. https://doi.org/10.17073/0368-0797-2023-1-35-42