Scroll to:
Strontium effect on the nature of phase equilibria in liquid metal containing calcium and aluminum
https://doi.org/10.17073/0368-0797-2022-12-895-903
Abstract
The use of complex strontium-containing alloys with alkaline earth metals for ladle refining of steel allows the efficiency of steel refining and modifying to be improved. Based on binary state diagrams of double systems SrO – CaO, SrO – Al2O3 , Al2O3 – CaO and data on the possibility of formation of solid solutions, the state diagram of the SrO – Al2O3 – CaO system in the temperature range of 1600 – 2600 °С was simulated. Theories of perfect solutions (for solid solutions of strontium and calcium aluminates), regular solutions (for solid oxide solutions) and subregular ionic solutions (for oxide melt) were used to build the liquidus lines. The thermodynamic analysis of the Fe – Sr – Ca – Al – O system as applicable to steel refining processes with calcium and strontium alloys at 1600 °С was carried out. Simulation results show that the complex mechanism of interaction of active elements with oxygen will be implemented in the process of refining steel deoxidized with aluminum. In this case calcium and strontium interaction with oxygen occurs both for elements dissolved in iron, and at the boundary of the gas phase containing calcium and strontium with molten liquid iron. The interaction of calcium and strontium with oxygen in the presence of aluminum (0.05 %) results in a high probability of formation of SrO – Al2O3 – CaO liquid oxide melts. This greatly facilitates the removal of reaction products from the melt. The resulting non-metallic inclusions are most likely complex calcium and strontium aluminates which are easily assimilated by slag due to the presence of strontium. The formation of undesirable corundum inclusions when treating metal with complex alloys containing strontium and calcium is unlikely.
For citations:
Mikhailov G.G., Makrovets L.A., Bakin I.V. Strontium effect on the nature of phase equilibria in liquid metal containing calcium and aluminum. Izvestiya. Ferrous Metallurgy. 2022;65(12):895-903. https://doi.org/10.17073/0368-0797-2022-12-895-903
Introduction
Reducing the contamination of steel with nonmetallic inclusions (NMI) can significantly improve the operational properties of steel products. One of the issues requiring further study is optimization of the processes related to refining of liquid metal in the process of ladle refining of steel. The efficiency and cost-effectiveness of producing critical steel depends primarily on the properties of the materials used, allowing control of the physical and chemical state of the metallic melt [1].
The application of calcium-containing materials for modification helps to control the degree of metal contamination by NMI, as well as the composition and shape of non-metallic inclusions. The use of calcium silicon and ferric calcium in ladle refining is associated with a number of process difficulties. Calcium exhibits a tendency to evaporation and secondary oxidation. This during steel crystallization results in its deficiency and activation of the formation of obstinate refractory calcium aluminates and stringer inclusions of Al2O3 . With low and unstable assimilation of calcium by the metal, ensuring the optimal [Ca]/[Al] ratio, and, therefore, obtaining confidently high steel quality is a difficult task [2].
At the current time, modifiers containing strontium along with calcium are increasingly being used for refining the metal deoxidized with aluminum. The influence of strontium additives on the processes of liquid steel deoxidation and modification is actively discussed in scientific literature. The application of alloys containing the alkaline earth metals (AEM) complex for processing of 17G1S-U steel helped to improve the purity of the metal in terms of NMI, as well as corrosion resistance and impact strength [3]. The use of strontium-containing modifiers promotes the refinement of both the metal structure and NMI. This results in an increase in the mechanical characteristics of castings [4 – 6]. Data on the use of strontium as a refining agent is also presented in [7, 8]. Some literature data on phase formation and thermodynamics of elements interaction in liquid iron [15 – 18] is available for the Fe – Al – Ca – O [9 – 12] and Fe – Al – Sr – O [13, 14] systems. The Fe – Sr – Ca – Al – O system, for which thermodynamic parameters are absent in the literature, is also of considerable interest.
The aim of the work is to study thermodynamic analysis of the Fe – Sr – Ca – Al – O system during liquid iron refining with calcium and strontium alloys at a steelmaking temperature of 1600 °С.
Simulation method and results
In order to calculate the solubility surface of components in the metal (SSCM) of the Fe – Sr – Ca – Al – O system, thermodynamic data on the triple state diagram of the SrO – Al2O3 – CaO oxide system is required. In [17], the state diagram with only schematic division is given. The presence of solid solutions: calcium and strontium oxides and aluminates is shown. In [18] a calculated state diagram of this system was built on the basis of [17].
In this work, binary diagrams of the SrO – CaO [19], SrO – Al2O3 [20] and Al2O3 – CaO [21] state and data on the possibility of formation of solid solutions [17] were used to simulate the state diagram of the SrO – Al2O3 – CaO system in the temperature range of 1600 – 2600 °С. Theories of perfect solutions (for solid solutions of strontium and calcium aluminates), regular solutions (for solid oxide solutions) and subregular ionic solutions (for oxide melt) were used to build the liquidus lines.
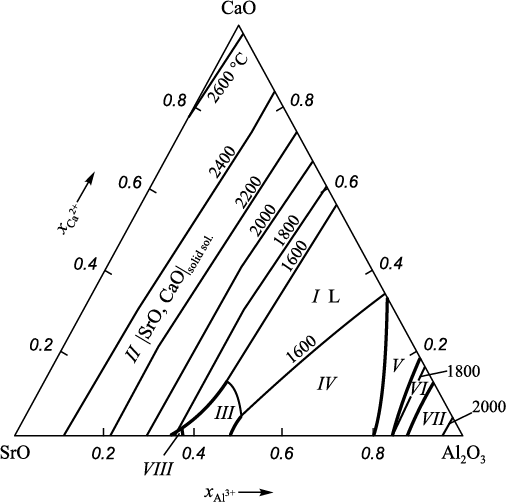
This paper presents the calculated state diagram (Fig. 1) of the SrO – Al2O3 – CaO system. The diagram consists of eight stability areas of the following phases: I is the area of liquid oxides (SrO, Al2O3 , CaO); II is the area of solid solution of |SrO, CaO| with unlimited solubility in each other; III is the area of solid solution of (Sr, Ca)3Al2O6 based on strontium aluminate; IV, V and VI are the areas of solid solutions of strontium and calcium mono-, bi- and hexaaluminates; VII is the area of corundum; and VIII is the area of Sr4Al2O7 . No ternary compounds were detected in the system in question. Wide areas of solid solutions (oxides and various calcium and strontium aluminates) can be seen in the diagram.
Fig. 1. Calculated diagram of the SrO – Al2O3 – CaO system |
In Table 1 (K is a melt constant of oxides and their compounds) thermodynamic data are given, used to calculate the state diagram of the SrO – Al2O3 – CaO system. Straight brackets in this table correspond to solid oxides and compounds, and parentheses correspond to oxide melt components.
Table 1. Thermodynamic data for phase transition reactions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The theory of subregular ionic solutions, taking into account the dependence of the coordination number on the slag composition, and the method of selecting the energy parameters for an oxide melt are described in [21]. Table 2 shows the energy parameters of the theory of subregular ionic solutions for the SrO – Al2O3 – CaO oxide system. The activities of the SrO – CaO solid solution components were calculated using the theory of regular solutions [21] (the energy parameter of the theory is 28,568 J/mol [19]). The activities of strontium and calcium aluminate solid solutions components were equated to their mole fractions [21].
Table 2. Parameters of the theory of subregular ionic solutions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
When considering the interaction of calcium, strontium, and aluminum with oxygen in liquid iron, double and triple oxide state diagrams (Table 2) need to be considered. However, the main diagram determining the non-metallic inclusions in the system under consideration will be the SrO – Al2O3 – CaO diagram (Fig. 1).
Table 3 shows all possible reactions which can occur in the Fe – Sr – Ca – Al – O system. However, only some reactions are possible depending on the composition of the liquid metal, temperature and total pressure. Square brackets in the table correspond to metallic melt, curved brackets correspond to the gas phase, and parentheses and straight brackets correspond to oxide and metallic melts. Temperature dependences of the equilibrium constant of chemical reactions taking place in the system under study are also given.
Table 3. Temperature dependences of equilibrium constants of chemical reactions
|
Strontium and calcium oxides form a continuous series of solid solutions [19], and iron oxide FeO dissolves only in oxide CaO (not more than 0.05 at 1600 °C). For the FeO in CaO solution, the energy parameter of the theory of regular solutions is 33,362 J/mol.
Table 4 lists the first-order interaction parameters required to calculate the activity of metallic melt components.
Table 4. Parameters of interaction of liquid iron components \(e_i^j\) at 1600 °C
| ||||||||||||||||||||||||||||||||||||||||||||
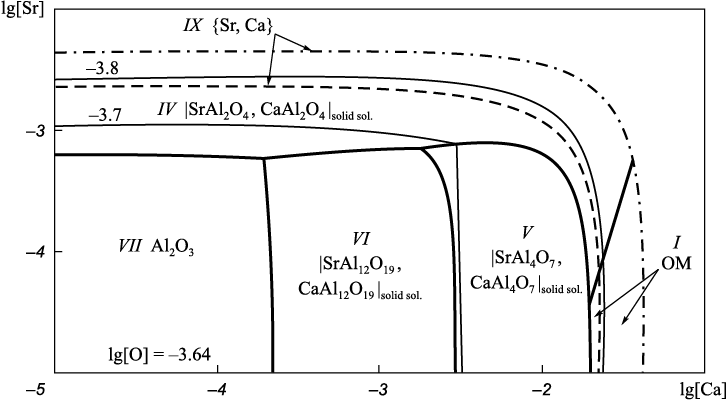
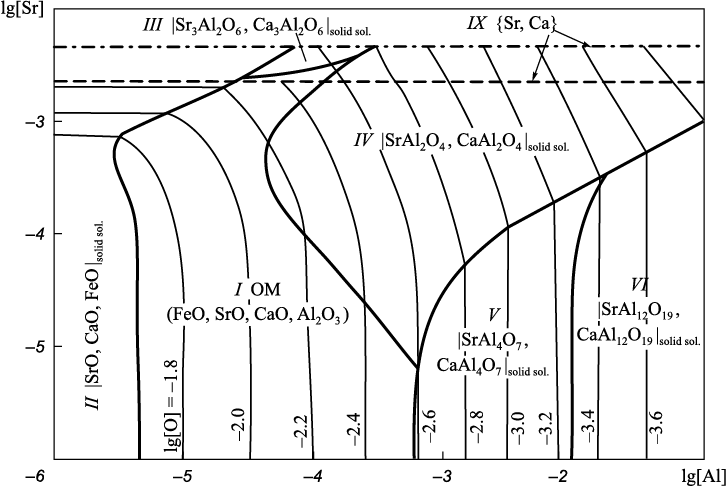
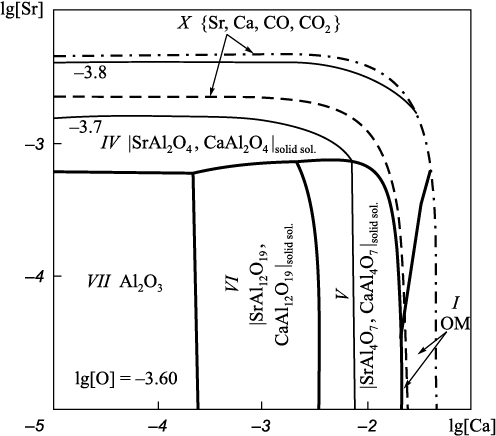
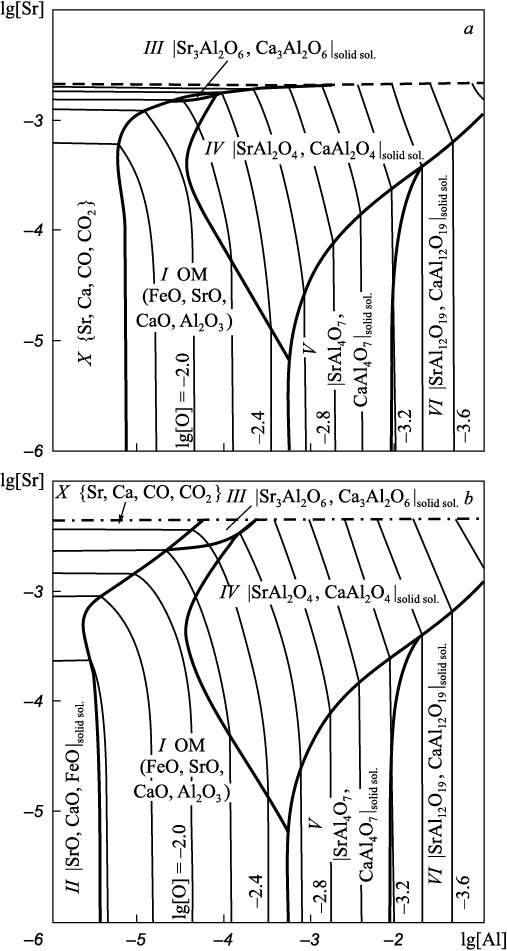
Projection of the solubility surface on the plane can be built only for two components (oxygen content is given by iso-oxygen sections), therefore, one of the components of the metal melt in the Fe – Sr – Ca – Al – O system shall be recorded. In this work we performed calculations for two variants: the aluminum (Fig. 2) or calcium (Fig. 3) concentration is recorded at a temperature of 1600 °С; and the total pressure of 101.3 and 202.6 kPa (dashed and dash-dotted lines). When calculating the Fe – Sr – Ca – Al – O – С system, carbon content must also be recorded (Fig. 4, 5). The final composition of the metal after the completion of the refining process is plotted on the solubility surface of components in the metal (SSCM). The thin lines are iso-oxygen sections of the solubility surface. The contrasting lines show compositions of the liquid metal in equilibrium with two oxide phases. The areas bounded by contrasting lines show compositions of liquid metal in equilibrium with one oxide phase. Area I shows the compositions of liquid metal in equilibrium with oxide melt (OM); II – with solid oxide solutions; III – with solid solution (Sr, Ca)3Al2O6 based on strontium aluminate; IV, V and VI – with solid solutions of strontium and calcium mono-, bi- and hexaaluminates; VII – with corundum; IX – with the gas phase {Sr, Ca}; X – with the gas phase {Sr, Ca, CO, CO2 } with traces of CO, CO2 ; XI – with the gas phase {Sr, Ca, CO, CO2 } of variable composition. All numbers of areas in Fig. 1 (except VIII) and in Fig. 2 – 5 correspond to each other. Area VIII in Fig. 2 – 5 is absent, since this area should be marked with compositions of metal in equilibrium with Sr4Al2O7 . However, its formation at given calcium or aluminum concentrations and temperature of 1600 °С is unlikely.
Fig. 2. SSCM of the Fe – Sr – Ca – Al – O system (t = 1600 °С, [Al] = 0.05 %)
Fig. 3. SSCM of the Fe – Sr – Ca – Al – O system (t = 1600 °С, [Ca] = 0.001 %)
Fig. 4. SSCM of the Fe – Sr – Ca – Al – O – С system (t = 1600 °С, [Al] = 0.05, [С] = 0.1 %)
Fig. 5. SSCM of the Fe – Sr – Ca – Al – O – С system |
In Fig. 2 – 4 and 5, b the gas phase area is projected onto a line, as it is located perpendicular to the plane of the figures. In Fig. 5, a (202.6 kPa) the area of liquid metal compositions in equilibrium with the gas phase is quite wide. Table 5 shows the contents of strontium, aluminum and oxygen in liquid iron and their corresponding partial pressures {Sr, Ca, CO, CO2 }. It can be seen that composition of the gas phase varies from 96.24 kPa for CO to 96.24 kPa for strontium. At the same time, the calcium pressure in the gas mixture remains almost constant (approximately 4.05 kPa), regardless of the strontium and aluminum concentrations.
Table 5. Composition of liquid metal and gas phase (Fig. 5, a, [Ca] = 0.001 %)
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The formation of aluminate solid solution based on Sr3Al2O6 (area III) is possible only at elevated pressure (above 1 atm) and strontium concentrations above 0.003 % (hereinafter by weight) in the Fe – Sr – Ca – Al – O system (Fig. 3). In the presence of minimum carbon concentrations (0.1 %) in the system under study, it is also the solid solution under study (Fig. 5, a) cannot be formed at atmospheric pressure but possible at increased pressure (Fig. 5, b).
Calcium monoaluminate has a melting point of 1601 °С [21], so in practice it does not represent non-metallic inclusions. However, in the presence of strontium, it appears on the SSCM of the Fe – Sr – Ca – Al – O system (Fig. 2 – 5) as a solid solution with strontium aluminate. According to the calculations, the formation of strontium and calcium mono- and bialuminates is most likely.
The possibility of formation of liquid oxide inclusions deep in the metal (pressure over 101.3 kPa) is very likely. This allows prediction of high refining properties of complex alloys with calcium and strontium in the metal deoxidized with aluminum.
Conclusion
In the process of refining steel deoxidized with aluminum, the complex mechanism of interaction of the active elements with oxygen is likely. Calcium and strontium interaction with oxygen occurs both for elements dissolved in iron and at the boundary of the gas phase containing calcium and strontium with the liquid iron melt. The interaction of calcium and strontium with oxygen with the aluminum content typical for industrial technologies may result in the formation of liquid oxide melts SrO – Al2O3 – CaO. This greatly facilitates the removal of reaction products from the melt. The NMI thus formed are most likely complex calcium and strontium aluminates, which are easily removed from the melt due to the presence of strontium. The formation of undesirable corundum Al2O3 inclusions when treating metal with complex alloys with strontium and calcium is unlikely. The thermodynamic analysis of processing steel deoxidized with aluminum by complex alloys containing calcium and strontium allows predicting the high refining capacity of such alloys.
References
1. Ryabchikov I.V., Mizin V.G., Usmanov R.G., Golubtsov V.A., Milyuts V.G. Criteria for assessing the quality of deoxidizers and modifiers for steel. Stal’. 2015, no. 2, pp. 24–27. (In Russ.).
2. Reformatskaya I.I., Rodionova I.G., Beilin Yu.A., Nisel’son L.A., Podobaev A.N. The effect of nonmetal inclusions and microstructure on local corrosion of carbon and low-alloyed steels. Protection of Metals. 2004, vol. 40, no. 5, pp. 447–452. https://doi.org/10.1023/B:PROM.0000043062.19272.c5
3. Bakin I.V., Shapovalov A.N., Kuznetsov M.S., Shaburova N.A., Usmanov R.G., Golubtsov V.A., Ryabchilov I.V., Mizin V.G., Panov V.N. Industrial tests of microcrystalline complex alkaline earth metal alloys when casting pipe steel. Steel in Translation. 2020, vol. 50, no 11, pp. 795–800. https://doi.org/10.3103/S0967091220110030
4. Skok Yu.Ya. Investigation of deoxidizing ability of complex alloys containing alkaline earth metals and rare earth metals. Protsessy lit’ya. 2010, vol. 81, no. 3, pp. 8–12. (In Russ.).
5. Provorova I.B., Rozenberg E.V., Baranovskii K.E., Volosatikov V.I., Rozum V.A., Karas’ A.N., Chernyavskii M.S. Modifier for out-of-furnace treatment of steel containing alkaline earth metals. Lit’e i metallurgiya. 2016, vol. 83, no. 2, pp. 14–18. (In Russ.).
6. Golubtsov V.A., Ryabchikov I.V., Sumin S.I. Non-metallic inclusions → modification → metal quality. In: Actual Tasks of Physical Metal Science of Steels and Alloys. Materials of the XXIV Ural School of Metal Scientists-Heat-Treaters (March 19–23, 2018, Magnitogorsk). Chukin M.V., Emelyushin A.N. eds. Magnitogorsk: MSTU im. G.I. Nosova, 2018, pp. 222–229. (In Russ.).
7. Ryabchikov I.V., Panov A.G., Kornienko A.E. Characteristics of modifiers. Steel in Translation. 2007, vol. 37, no 6, pp. 516–521. https://doi.org/10.3103/S0967091207060113
8. Bakin I.V., Mikhailov G.G., Golubtsov V.A., Ryabchikov I.V., Dresvyankina L.E. Methods for improving the efficiency of steel modifying. Materials Science Forum. 2019, vol. 946, pp. 215–222. https://doi.org/10.4028/www.scientific.net/MSF.946.215
9. Wang L., Li J., Yang S., Chen C., Jin H., Li X. Coarsening behavior of particles in Fe–O–Al–Ca melts. Scientific Reports. 2019, vol. 9, article 3670. https://doi.org/10.1038/s41598-019-40110-x
10. Jung I.-H., Decterov S.A., Pelton A.D. A thermodynamic model for deoxidation equilibria in steel. Metallurgical and Materials Transactions B. 2004, vol. 35, no. 3, pp. 493–507. https://doi.org/10.1007/s11663-004-0050-4
11. Taguchi K., Ono-Nakazato H., Usui T., Marukawa K., Katogi K., Kosaka H. Complex deoxidation equilibria of molten iron by aluminum and calcium. ISIJ International. 2005, vol. 45, no. 11, pp. 1572–1576. https://doi.org/10.2355/isijinternational.45.1572
12. Cho S.-W., Suito H. Assessment of calcium-oxygen equilibrium in liquid iron. ISIJ International. 1994, vol. 34, no. 3, pp. 265–269. https://doi.org/10.2355/isijinternational.34.265
13. Korogodskaya A.N., Shabanova G.N. Thermodynamic database of refractory strontium aluminates. Zbіrnik naukovikh prats’ PAT “UkrNDІVognetrivіv im. A.S. Berezhnogo”. 2012, no. 112, pp. 208–213 (In Russ.).
14. Kalinina N.E., Kavats O.A., Fedyuchuk A.K. Microalloying with strontium of cast aluminum alloys used in rocket and space industry. Vestnik dvigatelestroeniya. 2006, no. 1, pp. 147–149. (In Russ.).
15. Schürmann E., Braun U., Pluschkell W. Investigations on the equilibria between Al–Ca–O containing iron melts and CaO–Al2O3–FeOn slags. Steel Research. 1998, vol. 69, no. 9, pp. 355–358. https://doi.org/10.1002/srin.199805564
16. Zheng H.-Y., Guo S.-Q., Qiao M.-R., Qin L.-B., Zou X.-J., Ren Z.-M. Study on the modification of inclusions by Ca treatment in GCr18Mo bearing steel. Advances in Manufacturing. 2019, vol. 7, no. 4, pp. 438–447. https://doi.org/10.1007/s40436-019-00266-1
17. Massazza F., Sirchia E. Equilibriums at the temperature of fusion in the ternary system SrO–Al2O3–CaO. Annali di Chimica. 1959, vol. 49, pp. 1352–1370.
18. Kuroki T., Saito Y., Matsui T., Morita K. Evaluation of phase diagrams for the SrO–Al2O3–CaO system by in-situ observation using confocal laser microscope. Materials Transactions. 2009, vol. 50, no. 2, pp. 254–260. https://doi.org/10.2320/matertrans.mra2008352
19. Mikhailov G.G., Vyatkin G.P., Makrovets L.A., Samoilova O.V., Bakin I.V. Thermodynamic analysis of the interaction processes of components in the Fe–Sr–Ca–O–C system under the conditions of the metal melt existence. Vestnik YuUrGU. Seriya “Metallurgiya”. 2020, vol. 20, no. 4, pp. 5–13. (In Russ.).
20. Makrovets L.A., Samoilova O.V., Bakin I.V. Thermodynamic assessment of phase equilibria in the SrO–Al2O3 system. Defect and Diffusion Forum. 2021, vol. 410, pp. 725–729. https://doi.org/10.4028/www.scientific.net/DDF.410.725
21. Mikhailov G.G., Leonovich B.I., Kuznetsov Yu.S. Thermodynamics of Metallurgical Processes and Systems. Moscow: MISiS, 2009, 520 p. (In Russ.).
22. Kubaschewski O., Alcock C.B. Metallurgical Thermochemistry. Oxford, New York: Pergamon Press, 1967. (Russ. ed.: Kubaschewski O., Alcock C.B. Metallurgicheskaya termokhimiya. Moscow: Metallurgiya, 1982, 392 p.).
23. Mikhailov G.G., Makrovetz L.A., Samoilova O.V., Smirnov L.A. Phase equilibria in the liquid steel deoxidized with aluminum and calcium in the presence of magnesium. Russian Metallurgy (Metally). 2020, vol. 2020, no. 6, pp. 640–648. https://doi.org/10.1134/S0036029520060130
24. Makrovets L.A., Samoilova O.V., Mikhailov G.G., Bakin I.V. Thermodynamic analysis of strontium deoxidizing ability in liquid iron at presence of aluminum. Izvestiya. Ferrous Metallurgy. 2021, vol. 64, no. 10, pp. 768–777. (In Russ.). https://doi.org/10.17073/0368-0797-2021-10-768-777
25. Samoilova O.V., Makrovets L.A. Thermodynamic modeling of phase equilibria in the FeO–MgO–Al2O3 system. Materials Science Forum. 2020, vol. 989, pp. 3–9. https://doi.org/10.4028/www.scientific.net/msf.989.3
26. Fuwa T., Chipman J. The carbon-oxygen equilibria in liquid iron. Transactions of AIME. 1960, vol. 218, pp. 887–891.
27. Sigworth G.K., Elliott J.F. The thermodynamics of liquid dilute iron alloys. Metal Science. 1974, vol. 8, no. 1, pp. 298–310. https://doi.org/10.1179/msc.1974.8.1.298
28. Park J. H., Todoroki H. Control of MgO·Al2O3 spinel inclusions in stainless steels. ISIJ International. 2010, vol. 50, no. 10, pp. 1333–1346. https://doi.org/10.2355/isijinternational.50.1333
29. Faulring G.M., Ramalingam S. Inclusion precipitation diagram for the Fe–O–Ca–Al system. Metallurgical Transactions B. 1980, vol. 11, no. 1, pp. 125–130. https://doi.org/10.1007/BF02657181
About the Authors
G. G. MikhailovRussian Federation
Gennadii G. Mikhailov, Dr. Sci. (Eng.), Prof. of the Chair of Materials Science and Physical Chemistry of Materials
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
L. A. Makrovets
Russian Federation
Larisa A. Makrovets, Engineer of the Chair of Materials Science and Physical Chemistry of Materials
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
I. V. Bakin
Russian Federation
Igor’ V. Bakin, Head of the Division of Innovation, Modernization and Technical Development, LLC RPE Technology, Lecturer of the Chair of Materials Science and Physical Chemistry of Materials, South Ural State University
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
25 Vodrem Vil. – 40, Chelyabinsk 454901, Russian Federation
Review
For citations:
Mikhailov G.G., Makrovets L.A., Bakin I.V. Strontium effect on the nature of phase equilibria in liquid metal containing calcium and aluminum. Izvestiya. Ferrous Metallurgy. 2022;65(12):895-903. https://doi.org/10.17073/0368-0797-2022-12-895-903