Scroll to:
Removal of chlorine from electric arc furnace dust by oxidative roasting
https://doi.org/10.17073/0368-0797-2025-5-543-549
Abstract
The growth of steel production and consumption leads to the formation of a large amount of technogenic waste. One of the wastes is electric arc furnace (EAF) dust. In the Russian Federation, about 0.7 million tons of dust are annually generated. The paper studies the dust of one of the metallurgical enterprises, in which zinc is mainly contained in the form of ZnFe2O4 , and also contains harmful compounds of Cl and Pb, which reduce the quality of Waelz oxide during subsequent processing. The studied dust was subjected to high-temperature oxidative roasting in a muffle furnace. The experiments were carried out in the temperature range of 300 – 1100 °C with roasting time of 1 h. In the temperature range of 900 – 1100 °C, the roasting time varied within 3 – 9 h. The composition was determined using XRD phase analysis and micro-X-ray spectral method. It was found that at temperature of 900 °C and roasting time of 9 h the degree of Cl removal reaches 78 %. At temperature of 1000 ℃ and roasting time of 9 h, the degree of Cl removal reaches 99.4 % with Zn losses of 19.8 %. At temperature of 1100 ℃ and roasting time of 3 h the degree of Cl removal is 91.2 %, and Zn losses reach 37.8 %; thereby, carrying out the oxidative roasting at this temperature is impractical. Experimental studies have shown that it is possible to effectively remove chlorine from EAF dust which predominantly contains zinc in the form of ZnFe2O4 using high-temperature oxidative roasting with relatively low zinc losses in the temperature range of 900 – 1000 °C.
Keywords
For citations:
Grigor’ev E.V., Kapelyushin Yu.E., Bil’genov A., Stepanov D.V., Khalikulov A.A. Removal of chlorine from electric arc furnace dust by oxidative roasting. Izvestiya. Ferrous Metallurgy. 2025;68(5):543-549. https://doi.org/10.17073/0368-0797-2025-5-543-549
Introduction
The continuous growth of steel production and consumption inevitably leads to the generation of large amounts of technogenic waste. On average, 25 – 30 kg of electric arc furnace (EAF) dust is produced per ton of steel. In the Russian Federation, approximately 0.7 million tons of this dust are generated every year. It is typically stored in dumps, resulting in the loss of valuable metals such as iron, zinc, and lead1 [1; 2]. The accumulation of such waste poses a potential hazard to both ecosystems and human health [3]. The chemical composition of EAF dust varies depending on the specific production technology. The zinc content ranges from 2 to 25 %, and in some cases reaches up to 40 %. Understanding the physicochemical behavior of EAF dust components with the aim of extracting zinc, lead, and iron, as well as removing chlorine, is an important objective of modern metallurgical production [4 – 6].
In industrial practice, EAF dust is most often treated using pyrometallurgical methods. The Waelz process remains the predominant technology, accounting for about 80 % of all processed dust [7]. EAF dust is composed mainly (up to 90 %) of oxides, while the remaining portion consists of ferrites, sulfates, and chlorides, including sodium chloride (NaCl), potassium chloride (KCl), and chlorides of zinc and lead [8]. One of the main challenges in EAF dust recycling is its high chloride content. These chlorides originate from chlorine-containing compounds present in the steel scrap, such as polymer materials and paint coatings.
Roasting is one of the approaches used to remove contaminants from EAF dust. In studies [9; 10], oxidative roasting was performed at 950 °C with additional air supply. According to the results, about 98 % Pb and Cl and 1 % Zn volatilized. Other studies [11 – 13] investigated roasting in various gaseous atmospheres – air, CO2 , and SO2 . The dust samples were heated to 200 – 600 °C and held for 1 – 5 h. The authors selected low-temperature conditions to minimize zinc losses that occur at elevated temperatures. The most effective atmospheres were CO2 and SO2 , whereas roasting in air showed the lowest efficiency. Sulfatizing roasting reduced the chloride content by 83 % (from 70.2 to 12.1 mg/kg), while carbonation roasting achieved an 81 % reduction (from 70.20 to 13.23 mg/kg). In another study [14], EAF dust was roasted with CaO added to convert zinc ferrite into zinc oxide. The experiments were conducted at 1100 °C for 3 h, and it was found that approximately 98 % Cl and Pb were removed from the initial dust. In [15], roasting was carried out in a muffle furnace at temperatures ranging from 300 to 1150 °C. The results showed that heating the dust to 1150 °C completely eliminated sodium and chlorine, while potassium and lead contents decreased by 81 and 83.5 %, respectively. Zinc losses did not exceed 5 %. Chlorine removal results by roasting were also reported in [16]. Crucibles containing dust were heated at a rate of 300 °C/h to 900, 1000, and 1100 °C with a roasting time of 240 min. After roasting, the chlorine content in the sinter decreased from 3.02 to 0.01 – 0.02 %, corresponding to a chlorine removal degree of 99.6 %. However, no data on zinc losses were provided.
Overall, published studies report inconsistent results concerning the efficiency of oxidative roasting in air atmosphere, especially with respect to zinc losses. Therefore, the aim of this study was to further evaluate the effectiveness of oxidative roasting in air atmosphere for chlorine removal from EAF dust at high temperatures.
Initial dust and experimental procedure
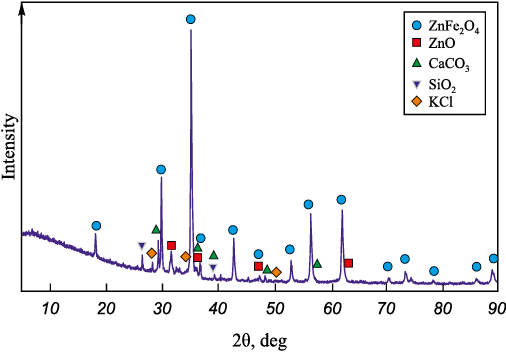
The study investigated electric arc furnace (EAF) dust obtained from one of the metallurgical enterprises. A representative sample for chemical and phase composition analysis was prepared by homogenizing the collected dust. The phase composition of the dust was determined by X-ray diffraction (XRD) using a Rigaku Ultima IV diffractometer. The data were processed with the Match software package. The main identified phases (%) were as follows: ZnFe2O4 – 69; ZnO – 6; CaCO3 – 17; SiO2 – 5; and KCl – 3.
Fig. 1. Phase composition of the researched EAF dust |
The elemental chemical composition of the researched dust is presented in Tables 1 and 2. The composition was determined by micro-X-ray spectral analysis using a JEOL JSM-7001F scanning electron microscope. Statistical errors were evaluated using mathematical methods. To obtain statistically reliable data, nine samples (portions of the homogenized EAF dust) were collected from a well-mixed bulk container. For each sample, four spectra were recorded (area analysis at 100× magnification). Mean values of the analyzed elements were calculated from 36 spectra, and the confidence interval radius was determined using the SPSS software. The resulting data are shown in Table 1.
Table 1. Composition of the researched EAF dust (statistical data, wt. %)
Table 2. Average composition of the researched EAF dust, wt. %
|
A series of oxidative roasting experiments was carried out in a muffle furnace. Each 18 g dust sample was placed in a corundum crucible and loaded into the furnace preheated to the desired temperature. The experiments were conducted in the temperature range of 300 – 1100 °C with a roasting time of 60 min under an air atmosphere.
Results and discussion
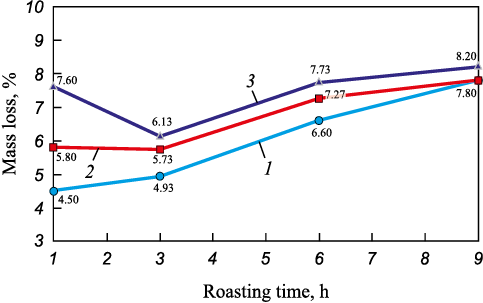
Fig. 2 illustrates the effects of temperature and time on the mass change of EAF dust samples during oxidative roasting.
Fig. 2. Effects of time and temperature on mass change |
As seen in Fig. 2, an increase in temperature causes slight mass variations of the samples (within 1 – 3 %), both upward and downward. These fluctuations are likely associated with the decomposition of carbonates and hydroxides, the volatilization of certain elements and compounds, as well as the further oxidation of metals to higher oxides.
After roasting, the dust samples were examined under an electron microscope. The results are presented in Table 3
Table 3. Composition of the EAF dust after oxidative roasting
|
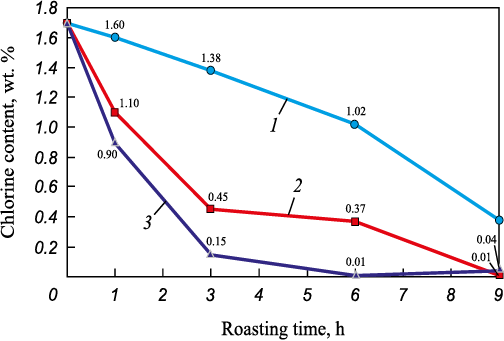
As shown in Table 3, an increase in temperature leads to a decrease in the chlorine content of the EAF dust samples. Based on these findings, additional high-temperature oxidative roasting experiments were carried out with varying roasting times to evaluate the effect of this parameter on dust dechlorination. Dust samples weighing 10 g were placed in corundum crucibles and loaded into a muffle furnace. The experiments were conducted at temperatures ranging from 900 to 1100 °C, with roasting times of 3, 6, and 9 h. After roasting, the samples were analyzed using electron microscopy. The experiments showed that the maximum degree of dechlorination reached approximately 98.9 % at 1100 °C and a roasting time of at least 6 h. At a roasting time of 9 h, a similar dechlorination degree of 96.8 % was obtained. The experimental results are shown in Fig. 3.
Fig. 3. Effects of time and temperature on Cl content in the EAF dust samples |
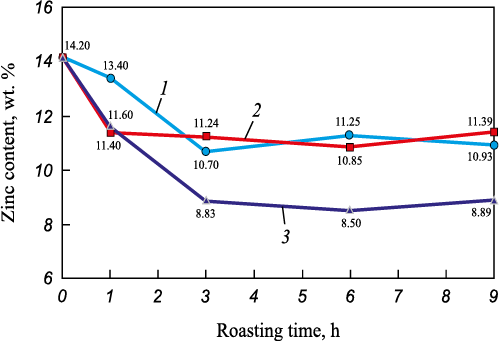
Further electron microscopy analysis revealed that oxidative roasting at 1100 °C for more than 6 h is inefficient because of increased zinc losses. At 1000 °C, zinc losses reached approximately 24.8 %, whereas at 1100 °C they increased to 38.5 %. These results are presented in Fig. 4.
Fig. 4. Effects of time and temperature on Zn content in the EAF dust samples |
The findings indicate that increasing temperature and roasting time during oxidative roasting significantly reduces the chlorine content in EAF dust, from 1.70 to 0.04 – 0.10 %. Temperature is therefore one of the most critical parameters influencing the efficiency of dechlorination. According to the data in Table 3, the maximum chlorine content (2.5 wt. %) was observed at 600 ℃, which can be attributed to minimal chlorine removal and to the decomposition of calcium hydroxide Ca(OH)2 and calcium carbonate CaCO3 . This temperature range corresponds to the decomposition temperatures of these compounds. A general increase in the concentrations of other elements in the dust was also noted. However, to confirm the relationship between these effects and the decomposition of hydroxides and carbonates, additional studies on phase transformations during heating are required. In the temperature range of 700 – 1100 °C, the chlorine content decreased from 2.5 to 0.9 wt. %, which agrees with the data reported in [15; 17 – 19]. Additional experiments were performed to assess the effect of roasting time at 900 – 1100 °C, varying from 1 to 9 h with 3 h intervals. As seen in Figs. 3 and 4, both chlorine and zinc losses increase with longer roasting time. Table 4 summarizes the obtained dechlorination degrees and zinc losses.
Table 4. Degree of chlorine removal and zinc loss at different temperatures
| |||||||||||||||||||||||||||||||||||||||||||||||
At a roasting temperature of 1000 °C and a roasting time of 3 h, the degree of chlorine removal was 73.5 %, while zinc losses remained within 20.8 %. Extending the roasting time to 9 h resulted in almost complete chlorine removal (97.4 %) with zinc losses of 19.8 %. At a roasting temperature of 1100 °C and a roasting time of 3 h, the degree of chlorine removal reached 91.2 %, but zinc losses increased significantly to 37.8 %. When the roasting time was increased to 6 h, chlorine removal was nearly complete (99.4 %), accompanied by similarly high zinc losses of approximately 40.1 %.
Conclusions
The literature provides conflicting information regarding the practicality of high-temperature oxidative roasting, mainly due to inconsistent data on zinc losses. However, the present experimental study has shown that effective chlorine removal from EAF dust – where zinc is predominantly present in the form of ZnFe2O4 – can be achieved. The main parameters influencing the efficiency of chlorine removal are the roasting temperature and roasting time. Maximum efficiency is reached at temperatures above 900 °C and roasting times longer than 3 h. At 1000 °C and a roasting time of 9 h, almost complete chlorine removal was achieved, while zinc losses reached about 20 wt. %.
Thus, high-temperature oxidative roasting at an optimal temperature of 900 – 1000 °C (to avoid excessive zinc losses) can be considered an effective method for EAF dust dechlorination and can be integrated into existing metallurgical production processes.
References
1. Leont’ev L.I., Dyubanov V.G. Technogenic wastes of ferrous and non-ferrous metallurgy and environmental problems. Ekologiya i promyshlennost’ Rossii. 2011;(4):32–35. (In Russ.).
2. Adilov G., Kareva N.T., Roshchin V.E. Influence of copper and silicon on phase transformations in the iron – carbon system. Izvestiya. Ferrous Metallurgy. 2024;67(1):73–75. (In Russ.). https://doi.org/10.17073/0368-0797-2024-1-73-75
3. Konakova A.G., Osipova E.A. Prevalence of zinc compounds in the environment and their role for living organisms. In: University Complex as a Regional Centre of Education, Science and Culture: Proceedings of the All-Rus. Sci.-Method. Conf. Orenburg, 2023. 2023:4384–4387. (In Russ.).
4. Adilov G., Povolotskii A.D., Roshchin V.E. Thermodynamic modeling of metal reduction in copper-smelting slags and experimental verification of its results. Izvestiya. Ferrous Metallurgy. 2022;65(8):581–589. (In Russ.). https://doi.org/10.17073/0368-0797-2022-8-581-589
5. Martins F.M., dos Reis Neto J.M., da Cunha C.J. Mineral phases of weathered and recent electric arc furnace dust. Journal of Hazardous Materials. 2008;154(1–3):417–425. https://doi.org/10.1016/j.jhazmat.2007.10.041
6. Simonyan L.M., Demidova N.V. Selective extraction of carbon-free zinc and lead from EAF-dust. Izvestiya. Ferrous Metallurgy. 2020;63(8):631–638. (In Russ.). https://doi.org/10.17073/0368-0797-2020-8-631-638
7. Ryazanov A.G., Koryagin Yu.D. Influence of heat treatment modes on the phase composition of zinc-containing materials: Final qualifying work. 2017:14–16. (In Russ.).
8. Grigor’ev E.V., Kapelyushin Yu.E. Manufacturing, curing and mechanical testing of BREX from electric arc furnace dust. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2023;79(4):334–339. (In Russ.).
9. Stovpchenko A.P., Kamkina L.V., Proidak Yu.S., Derevyanchenko I.V., Kucherenko O.L., Bondarenko M.Yu. Theoretical and experimental studies of composition and reducibility of dust of arc steelmaking furnaces. Electrometallurgy. 2009;(8):29–36. (In Russ.).
10. Doronin I.E. Industrial methods of steelmaking dust processing. Metallurg. 2010;(10):48–53. (In Russ.).
11. Yoo J.M., Kim B.S., Lee J.C., Kim M.S., Nam C.W. Kinetics of the volatilization removal of lead in electric arc furnace dust. Materials Transactions. 2005;46(2);323–328. https://doi.org/10.2320/matertrans.46.323
12. Zhu F., Takaoka M., Oshita K., Kitajima Y., Inada Y., Morisawa S., Tsuno H. Chlorides behavior in raw fly ash washing experiments. Journal of Hazardous Materials. 2010;178(1–3):547–552. https://doi.org/10.1016/j.jhazmat.2010.01.119
13. Chen W.S., Shen Y.H., Tsai M.S., Chang F.C. Removal of chloride from electric arc furnace dust. Journal of Hazardous Materials. 2011;190(1–3):639–644. https://doi.org/10.1016/j.jhazmat.2011.03.096
14. Chairaksa-Fujimoto R., Inoue Y., Umeda N., Itoh S., Nagasaka T. New pyrometallurgical process of EAF dust treatment with CaO addition. International Journal of Minerals, Metallurgy, and Materials. 2015;22:788–797. https://doi.org/10.1007/s12613-015-1135-6
15. Yakornov S.A. Technology of processing of zinc-containing dusts of arc steelmaking furnaces with obtaining zinc powder: Cand. Tech. Sci. Diss. 2024:81–82,90,93. (In Russ.).
16. Chen W.S., Chou W.S., Wu C.C., Tsai M.S. Removal of chloride from EAF-dust by reactive roasting at low temperature. 100th Annual Conf. and Exhibition of the Air and Waste Management Association 2007. ACE 2007, Air and Waste Management Association; 2007.
17. Simonyan L.M., Demidova N.V. Dioxins and furans’ behavior in the process of zinc and lead removing from EAF dust. Izvestiya. Ferrous Metallurgy. 2019;62(11):840–845. (In Russ.). https://doi.org/10.17073/0368-0797-2019-11-840-845
18. Demin A.V., Rozhkov A.I., Grudnitskii O.M., Nikolaev V.V., Feklistov A.V. Finding ways of recycling dust of arc steel furnaces at the Belarusian metallurgic plant. Vestnik KIGIT. 2014;2(44):40–49. (In Russ.).
19. Leclerc N., Meux E., Lecuire J.M. Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using mononitrilotriacetate anion and hexahydrated ferric chloride Journal of Hazardous Materials. 2002;91(1–3):257–270. https://doi.org/10.1016/S0304-3894(01)00394-6
About the Authors
E. V. Grigor’evRussian Federation
Evgenii V. Grigor’ev, Research Engineer of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
Yu. E. Kapelyushin
Russian Federation
Yury E. Kapelyushin, Senior Researcher of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
A. Bil’genov
Russian Federation
Arman Bil’genov, Senior Lecturer of the Chair “Pyrometallurgical and Foundry Technologies”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
D. V. Stepanov
Russian Federation
Dmitrii V. Stepanov, Research Engineer of Department of Scientific and Innovation Activities
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
A. A. Khalikulov
Russian Federation
Artur A. Khalikulov, Laboratory Assistant of Department of Scientific and Innovation Activities
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
Review
For citations:
Grigor’ev E.V., Kapelyushin Yu.E., Bil’genov A., Stepanov D.V., Khalikulov A.A. Removal of chlorine from electric arc furnace dust by oxidative roasting. Izvestiya. Ferrous Metallurgy. 2025;68(5):543-549. https://doi.org/10.17073/0368-0797-2025-5-543-549