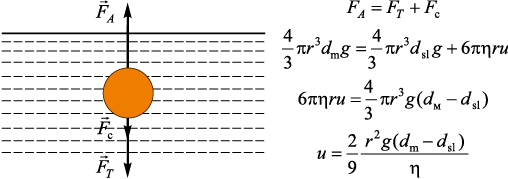Scroll to:
Physico-chemical analysis of 12Kh18N9TL steel melts for quality control of cast products
https://doi.org/10.17073/0368-0797-2025-5-534-542
Abstract
The authors studied the behavior of slag inclusions in four samples taken during casting of austenitic corrosion-resistant steel grade 12Kh18N9TL. The samples for the study were taken under production conditions after melting the charge (1), introducing Ti and FeMn, slag induction and additional loading (2), re-introducing Ti, slag induction (3), slag induction (4). The elemental composition and temperature of the melt were determined under production conditions. The physicochemical properties of the melts obtained from the selected samples were measured under laboratory conditions: surface tension and kinematic viscosity. The measurements were carried out in the temperature range from 1370 to 1760 °C in the mode of heating and subsequent cooling of the sample. When observing the sample during the measurement of surface tension, the release of slag inclusions from the volume of the drop occurs in the heating mode. During subsequent cooling of the formed drop of liquid steel, slag particles flow from the slag bath under the action of the Marangoni force. Analysis of the dependence of the slag particle ascent rate on its size showed that only particles up to 10 μm in size can remain in the melt volume, while larger particles have time to float to the surface of the liquid bath. It was found that slag particles up to 4 mm in size can flow onto the surface of the sample under the action of the Marangoni force. The volume fraction of slag inclusions was determined, and a correlation was established between it and the element composition of the sample. The authors made a conclusion about the effect of Ti additives in the melt as the cause of increase in the volume fraction of slag inclusions in the casting.
Keywords
For citations:
Shvetsov D.P., Chikova O.A., Tsepelev V.S., Sinitsin N.I., V’yukhin V.V. Physico-chemical analysis of 12Kh18N9TL steel melts for quality control of cast products. Izvestiya. Ferrous Metallurgy. 2025;68(5):534-542. https://doi.org/10.17073/0368-0797-2025-5-534-542
Introduction
Austenitic corrosion-resistant steel grade 12Kh18N9TL exhibits high mechanical properties combined with resistance to organic acid solutions [1]. The production of castings from 12Kh18N9TL steel is complicated by its low casting properties. The castings are often characterized by microstructural heterogeneity, susceptibility to intergranular corrosion, and low resistance to cracking. During foundry production, increased formation of films, ingot porosity, shrinkage cavities, and a high content of nonmetallic inclusions in the ingot structure are observed [2].
Modern quality requirements for cast products made of corrosion-resistant and heat-resistant steels such as 12Kh18N9TL are driven by their wide application in the power industry, chemical engineering, and aircraft manufacturing. Experimental and exploratory studies are carried out to improve the mechanical properties of cast products by stabilizing the austenitic structure through alloying and thermomechanical treatment [3 – 5]. In [6], the authors suggest that alloying with copper, silicon, titanium, and niobium favors achieving a combination of high corrosion-electrochemical and mechanical properties in steel. In [7], it was shown that laser pulse surface modification of stainless steel 12Kh18N10T produces iron oxide (Fe3O4 ) and chromium oxide (Cr2O3 ), with titanium dioxide (TiO2 ) also present [7]. The absence of undesirable surface contaminants, a decrease in their size and quantity, as well as an increase in the amount of retained austenite, enhance the corrosion resistance of austenitic steels [8]. It was established that in samples of austenitic stainless steels grades 03Kh18N10 and 08Kh18N10T, clusters of titanium nitrides and oxides of various dispersity are located around globular nonmetallic inclusions containing a secondary phase [9]. On the surface of cold-rolled sheet made of 08Kh18N10T steel, coarse defects in the form of delaminations were detected, consisting of titanium nitrides and a slag-forming mixture. Nonmetallic inclusions were noted to play a decisive role in degrading the mechanical properties of castings [10].
The casting properties of steel have a direct effect on obtaining castings and ingots with specified technological parameters. The most relevant physicochemical properties of the melt for foundry production are viscosity and surface tension. Slag induction during melting reduces the oxidation loss of volatile components and prevents the formation of oxide compounds. However, during melt tapping from the furnace, the slag becomes entrained in the melt, and slag inclusions may be present in steel ingots. In tests measuring the surface tension of liquid 12Kh18N9TL steel, the authors observed the release of slag during sample heating. Conducting systematic studies of the behavior of slag inclusions in steel is the objective of this work.
At “Precision Casting Center” (LLC, Ekaterinburg), castings of 12Kh18N9TL steel are produced as part of the import substitution program, highlighting the need to improve product quality at the melt preparation stage. Defects in finished products have been linked to the presence of slag inclusions in the casting. The objective of this study is to characterize the regularities of slag inclusion behavior in castings to improve control over the quality of 12Kh18N9TL cast products.
Materials and methods
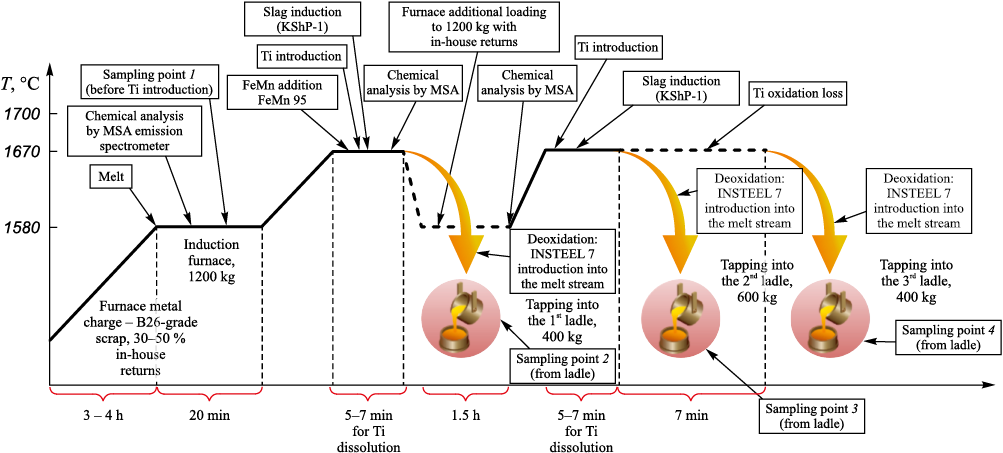
Samples of 12Kh18N9TL steel were taken from an induction crucible furnace under production conditions at “Precision Casting Center” (LLC, Ekaterinburg) according to the technological scheme shown in Fig. 1. The production process of cast products made of 12Kh18N9TL steel has the following specific features. As the metal charge, returns of the same grade from in-house production and B26-grade scrap are used. The melting of the metal charge lasts about 3.5 h, with heating of the melt to 1580 °C, at which point a sample is taken for elemental composition analysis. The elemental composition is determined using an MSA emission spectrometer (model MSA II v5). After titanium introduction, the melt surface was covered with the perlite-based slag coagulator KShP-1. The holding time for titanium dissolution in the melt is 5 – 7 min, and the melt tapping temperature into the ladle is 1650 – 1670 °C. Before melt tapping, a 200 g portion of aluminum is placed at the bottom of the ladle. The modifier INSTEEL 7 is introduced into the melt stream at a rate of 500 g per 400 kg of metal. After tapping the first portion of the melt into the ladle, the residual melt in the furnace is additionally loaded with 12Kh18N9TL steel and then heated to 1580 °C for 1.5 h, after which another sample is taken for elemental analysis. An 8 kg portion of titanium is added based on the elemental analysis results to achieve the required elemental composition. Before tapping the melt into the second and third ladles, slag induction on the melt surface is repeated, followed by heating to 1650 – 1670 °C. A 300 g portion of aluminum is also placed at the bottom of each ladle. During melt tapping, the modifier INSTEEL 7 is again introduced into the melt stream at a rate of 750 g per 600 kg of metal.
Fig. 1. Technological scheme of 12Kh18N9TL steel smelting with indication |
Thus, samples for the present study were taken under production conditions in accordance with the technological scheme shown in Fig. 1. Sample 1 was taken from the furnace before titanium introduction at a melt temperature of 1580 °C. Sample 2 was taken from the ladle after titanium introduction. Samples 3 and 4 were taken from the second and third ladles, respectively. The results of the chemical analysis of 12Kh18N9TL steel samples are presented in Table 1.
Table 1. Chemical composition of 12Kh18N9TL steel samples, wt. %
|
The kinematic viscosity (ν) and surface tension (σ) of liquid 12Kh18N9TL steel were measured using the equipment of the Research Center of Physics of Metallic Liquids, Institute of Physics of Metals (UrFU, Russia). The kinematic viscosity was measured by the oscillating-cup method [11; 12]. The tests were carried out in an atmosphere of high-purity helium under a pressure of 105 Pa. The temperature was measured with a BP-5/20 thermocouple, the readings of which were transmitted to a Termodat-14E2 controller. Kinematic viscosity was measured in 30 – 40 °C increments during heating and subsequent cooling. At each temperature, at least ten consecutive readings were acquired. Values were considered stabilized when the root-mean-square (RMS) deviation did not exceed the random measurement error. The oscillation parameters were recorded optically throughout the tests. The systematic error of the viscosity measurement was 3 %, and the random error governing within-test scatter did not exceed 1.5 % at the 95 % confidence level (p = 0.95).
The surface tension of liquid 12Kh18N9TL steel was measured by the sessile-drop method during heating to 1750 °C, followed by sample cooling. The working chamber was evacuated to 0.001 Pa and then backfilled with helium to approximately 105 Pa. Samples were held in the inert-gas chamber for 5 – 8 min at the melting temperature, then heated to 1750 °C in 30 – 40 °C increments. The isothermal hold at each setpoint was at least 15 min. The drop profile was recorded with a digital camera, and the images were processed on a computer. The geometric parameters of the drop profile were determined using SIAMS 700 image-analysis software with an accuracy of 0.3 – 0.6°. No melt evaporation or drop volume loss was observed. The measurement error for density and surface tension did not exceed 7 %, and the random error governing within-test scatter did not exceed 1.5 % at the 95 % confidence level (p = 0.95) [13 – 16]. The method and apparatus for determining the density and surface tension of metallic melts are described in [17 – 19].
Results and discussion
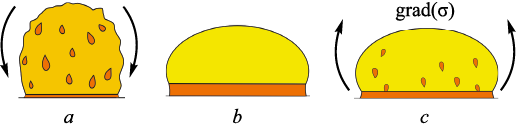
During observation of the sample with a digital camera in the course of surface tension measurements, slag release was detected during sample heating (Fig. 2, a), which made it impossible to perform measurements in the heating mode. When the maximum temperature was reached, the contour of the liquid drop was formed as the slag drained from the drop surface into the slag bath (Fig. 2, b). Upon subsequent cooling, slag flowed from the formed slag bath onto the sample surface (Fig. 2, c). These observations confirmed the presence of slag inclusions within the sample volume.
Fig. 2. Formation and evolution of slag inclusions on the sample surface |
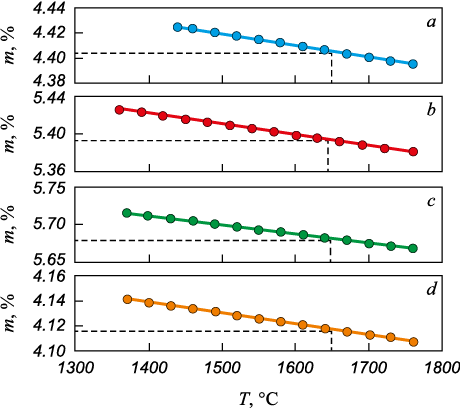
The volume fraction of slag in the sample was determined according to the procedure described in [20]. Fig. 3 shows the temperature dependence of the volume fraction of slag inclusions m (%) in the 12Kh18N9TL steel samples. A progressive increase in the volume fraction of slag inclusions from sample 1 to sample 3 is observed, whereas the lowest value was recorded for sample 4. The volume fractions of slag inclusions at the melting temperature of 12Kh18N9TL steel are given in Table 2.
Fig. 3. Temperature dependences of volume fraction of slag
Table 2. Volume fraction of slag inclusions, surface tension and kinematic viscosity
|
The volume fraction of slag inclusions increases from the first sample taken from the melt before titanium introduction to the third sample taken from the second ladle after furnace replenishment. In the fourth sample, taken from the third ladle according to the technological scheme (Fig. 1), the volume fraction of slag inclusions was minimal
A slag particle of radius rsl and density dsl located in the melt of density dm and dynamic viscosity μ, is affected by buoyancy force \({F_A} = \frac{4}{3}\pi {r^3}{d_{\rm{m}}}g,\) gravity \({F_T} = \frac{4}{3}\pi {r^3}{d_{{\rm{sl}}}}g\) and the Stokes force Fs = 6πrμu. Under the combined action of these forces, slag particles rise to the surface of the melt because of the density difference between the slag and the liquid metal (Fig. 4). When all three forces are balanced, a slag particle moves through the melt volume at a constant velocity u.
Fig. 4. Illustration of slag particles floating in the melt volume |
The ascent rate u of a slag particle depends on its size and can be expressed as
| \[u = \frac{{2{r^2}g({d_{\rm{m}}} - {d_{{\rm{sl}}}})}}{{9v{d_{\rm{m}}}}},\] | (1) |
where r is the particle radius; g is the acceleration due to gravity; dm is the density of the liquid metal; dsl is the slag density; v is the kinematic viscosity of the liquid metal.
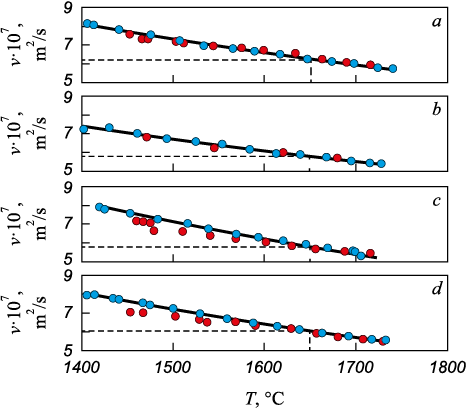
Fig. 5 presents the temperature dependence of kinematic viscosity obtained during heating and subsequent cooling. The absence of hysteresis between the heating and cooling branches indicates the lack of irreversible structural transformations in the melt, and consequently, the stability of slag particles within the melt volume up to 1740 °C. Therefore, slag particle removal from the melt is possible only due to their ascent to the surface.
Fig. 5. Temperature dependences of kinematic viscosity of liquid steel 12Kh18N9TL |
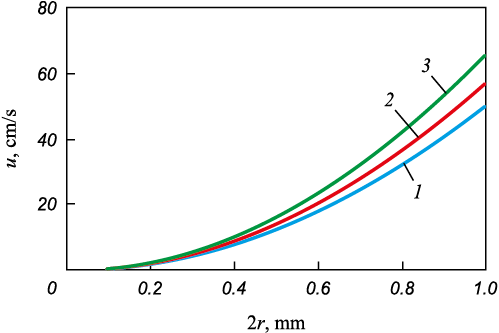
Fig. 6 shows the dependence of slag particle velocity in liquid 12Kh18N9TL steel on particle size, as calculated from Equation (1). It follows from Fig. 6 that large particles rise rapidly to the surface, while particles smaller than 10 μm fail to reach the surface during the smelting process and remain within the melt volume.
Fig. 6. Dependence of floating rate of slag particles |
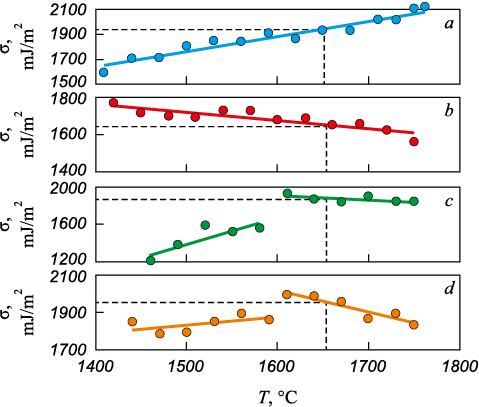
Fig. 7 displays the results of surface tension measurements for liquid 12Kh18N9TL steel samples. In the temperature dependences for samples 3 and 4, anomalies were detected near 1600 °C, associated with the formation of a slag film on the sample surface due to slag flow from the slag bath. Slag particles and liquid metal possess different surface tension values. The surface tension determines the force that pulls the drop toward an ellipsoidal shape. A surface tension gradient (dσ/dr) in the vertical direction induces the Marangoni force, which drives slag particles from the base of the drop toward its apex; this force is counterbalanced by gravity (Fig. 8). The Marangoni force was estimated from the surface tension gradient (dσ/dr) using expression [21]
| \[{F_{\rm{m}}} = 4\pi {r^2}\frac{{d\sigma }}{{dT}}\frac{{\partial T}}{{\partial r}} \Rightarrow {F_{\rm{m}}} = 4\pi {r^2}\frac{{d\sigma }}{{dr}}.\] | (2) |
Fig. 7. Temperature dependences of surface tension
Fig. 8. The Marangoni effect |
Thus, a slag particle located on the surface of a liquid 12Kh18N9TL steel drop is subject to both gravity Ff = ρsl gVp and the Marangoni force (2) (Fig. 8). The equilibrium condition of these forces can be written as:
\[\begin{array}{c}\frac{4}{3}\pi {r^3}{\rho _{{\rm{sl}}}}g = 4\pi {r^2}\frac{{d\sigma }}{{dr}},\\\frac{{d\sigma }}{{dr}} = {\rho _{{\rm{sl}}}}gr.\end{array}\]
From this, the expression for Δσ can be obtained \(\int {d\sigma } = \int {{\rho _{{\rm{sl}}}}grdr} ,\)
| \[\Delta \sigma = \frac{{{\rho _{{\rm{sl}}}}g{r^2}}}{2}.\] | (3) |
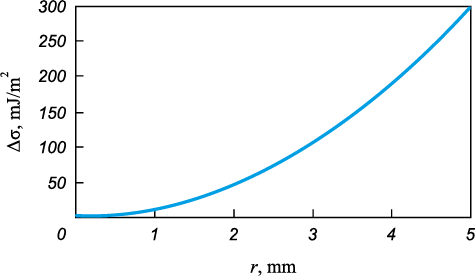
In equation (3), Δσ represents the difference between the surface tension values of slag and metal, at which the slag particle remains in equilibrium under the opposing actions of gravity and the Marangoni force. Fig. 9 shows the dependence of Δσ on the slag particle radius on the drop surface. When Δσ = 200 mJ/m2, slag particles larger than 4 mm can move upward from the base toward the apex of the drop surface. The observed slag flow phenomenon indicates that during slag bath formation at the base of the drop, the surface tension changes to a value at which the Marangoni forces become dominant.
Fig. 9. Dependence of Δσ on the radius r of a slag particle |
Table 3 presents the chemical composition of the samples and the corresponding volume fraction of slag inclusions. Analysis of the data shows that the volume fraction of slag inclusions increases with increasing titanium content, suggesting a decisive role of titanium additions in the formation of slag inclusions.
Table 3. Chemical composition of the samples, wt. % and volume fraction of inclusions
|
Conclusions
The behavior of slag inclusions was studied in samples taken during the casting of austenitic corrosion-resistant steel 12Kh18N9TL. In surface tension tests of liquid steel performed by the sessile-drop method using a digital camera, slag release from the sample was observed upon heating to 1760 °C. Slag drops flowed from the sample surface, forming a slag bath at the base of the drop. During subsequent cooling of the liquid-steel drop, slag particles were found to flow from the slag bath onto the sample surface under the action of the Marangoni force. The size dependence of the slag inclusion ascent rate and the Marangoni force magnitude was evaluated. Analysis of the relationship between slag particle ascent rate and particle size showed that only particles smaller than 10 μm can remain in the melt volume, while larger particles rise to the surface of the liquid bath. It was established that slag particles up to 4 mm in size can flow onto the sample surface under the action of the Marangoni force.
The physicochemical properties of liquid 12Kh18N9TL steel relevant to foundry practice, namely surface tension and kinematic viscosity, were measured over a temperature range of 1370 – 1760 °C. No hysteresis between the heating and cooling branches of the kinematic-viscosity curves was observed, indicating the absence of irreversible structural transformations in the melt. This suggests that slag particles remain stable in the melt up to 1760 °C. In the temperature dependences of surface tension for samples taken from the third and fourth ladles during 12Kh18N9TL steel casting, anomalies were detected near 1600 °C, corresponding to a sharp decrease in surface tension. Such anomalous behavior is attributed to slag particle flow from the formed slag bath onto the drop surface under the influence of the Marangoni force. The volume fraction of slag inclusions in the melt was also evaluated. The lowest volume fractions were recorded in the sample taken before titanium addition and in the sample taken from the fourth ladle.
The volume fraction of slag inclusions in the melt was also evaluated. The lowest volume fractions were recorded in the sample taken before titanium addition and in the sample taken from the fourth ladle. The reduction in slag inclusion volume fraction in the latter sample, taken from the final ladle into which the residual liquid metal was tapped from the furnace, is associated with slag particle flotation to the liquid-metal surface during steel smelting. A correlation was established between the volume fraction of slag inclusions and the titanium content in the samples. An increase in titanium concentration was accompanied by a rise in the volume fraction of slag inclusions, indicating that titanium plays a decisive role in the formation of slag inclusions. It was concluded that titanium additions to the melt lead to an increase in the volume fraction of slag inclusions in the casting.
References
1. Ul`yanin E.A. Corrosion-Resistant Steels and Alloys: Reference Book. Moscow: Metallurgiya; 1991:256. (In Russ.).
2. Ramazanov A.K., Ganeev A.A. Features of casting pipeline valve body parts made of corrosion-resistant steel 12Kh18N9TL. Vestnik of Nosov Magnitogorsk State Technical University. 2018;18(2):22–29. (In Russ.). https://doi.org/10.18503/1995-2732-2020-18-2-22-29
3. Makarov A.V., Skorynina P.A., Osintseva A.L., Yurovskikh A.S., Savrai R.A. Improving the tribological properties of austenitic 12Kh18N10T steel by nanostructuring frictional treatment. Metal Working and Material Science. 2015;69(4):80–92. (In Russ.). http://dx.doi.org/10.17212/1994-6309-2015-4-80-92
4. Voronenko B.I. Modern corrosion-resistant austenitic-ferritic steels (Review). Metal Science and Heat Treatment. 1997;(10):20–28. (In Russ.).
5. Tsukanov V.V., Tsyganko L.K., Shandyba G.A., Ziza A.I. Alloying and heat treatment effects on the properties of cast corrosion-resistant nitrogenous austenitic steel. Voprosy materialivedeniya. 2015;81(1):7–11. (In Russ.).
6. Fel’dgandler E.G., Plaskeev A.V. The alloying effect of Si and Cu on the mechanical and corrosive-electrochemical properties of austenite steel. Metallovedenie i termicheskaya obrabotka metallov. 2003;(10):12–21. (In Russ.).
7. Proskuryakov V.I., Rodionov I.V. Formation of the composition and characteristics of the surface of chromonicel steel 12CR18NI10T during laser modification in a layer of experimental alloying coating. Technical Physics. 2022;92(1): 84–91. https://doi.org/10.21883/JTF.2022.01.51856.173-21
8. Moradi M., Ghorbani D., Moghadam M.K., Kazazi M., Rouzbahani F., Karazi S. Nd:YAG laser hardening of AISI 410 stainless steel: Microstructural evaluation, mechanical properties, and corrosion behavior. Journal of Alloys and Compounds. 2019;795:213–222. https://doi.org/10.1016/j.jallcom.2019.05.016
9. Tokovoi O.K., Shaburov D.V. Study of nonmetallic phase in austenitic stainless steel. Izvestiya. Ferrous Metallurgy. 2014;57(12):20–24. (In Russ.). https://doi.org/10.17073/0368-0797-2014-12-20-24
10. Polonskii Ya.Ya., Bondareva O.P., Gonik I.L. Fractographic studies of metal from experimental melts of ferritic-austenitic steel 08Kh18G8N2T. Izvestiya Volgogradskogo gosuderstvennogo universiteta. 2011;5(78):142–144. (In Russ.).
11. Shvidkovskii E.G. Some Issues of Molten Metals Viscosity. Moscow: Gos. iz-vo tekhniko-teoreticheskoi literatury; 1955:206. (In Russ.).
12. Arsent’ev P.P., Yakovlev V.V., Krasheninnikov M.G., etc. Physicochemical Methods for Studying Metallurgical Processes. Moscow: Metallurgiya; 1988:511. (In Russ.).
13. Liggieri L., Passerone A. An automatic technique for measuring the surface tension of liquid metals. High Temperature Technology. 1989;7(2):82–86. https://doi.org/10.1080/02619180.1989.11753417
14. Egry I., Ricci E., Novakovic R., Ozawa S. Surface tension of liquid metals and alloys – Recent developments. Advances in Colloid and Interface Science. 2010;159(2):198–212. https://doi.org/10.1016/j.cis.2010.06.009
15. Ivashchenko Yu.N., Khilya G.P. Installation for measuring the free surface energy, contact angle and density of melts by the sessile drop method. Pribory i tekhnika eksperimenta. 1972;(6):208–211. (In Russ.).
16. Lee J., Kiyose A., Nakatsuka S., Nakamoto M., Tanaka T. Improvements in surface tension measurements of liquid metals having low capillary constants by the constrained drop method. ISIJ International. 2004;44(11):1793–1799. https://doi.org/10.2355/isijinternational.44.1793
17. Direktor L.B., Zaichenko V.M., Maikov I.L. An improved method of sessile drop for determining the surface tension of liquids. High Temperature. 2010;48(2):176–180. https://doi.org/10.1134/S0018151X10020069
18. Prasad L.C., Chatterjee S.K., Jha R.K. Atomic order and interionic pair potentials in Cu-Sn liquid alloys. Journal of Alloys and Compounds. 2007;441(1-2):43–51. https://doi.org/10.1016/j.jallcom.2006.09.079
19. Ricci E., Giuranno D., Grosso I., Lanata T., Amore S., Novakovic R., Arato E. Surface tension of molten Cu-Sn alloys under different oxygen containing atmospheres. Journal of Chemical and Engineering Data. 2009;54(6):1660–1665. https://doi.org/10.1021/je800717a
20. Gluzman L.D., E`del`man I.I. Laboratory control of coke production. Xar’kov: Gos. nauchno-tekhn. izd-vo literatury po chernij i tszvetnoi metallurgii; 1957:635. (In Russ.).
21. Zhao D., Gao J. Liquid phase separation in undercooled Cu–Co alloys under the influence of static magnetic fields. Philosophical Transactions of the Royal Society A. 2019;377:20180207. http://dx.doi.org/10.1098/rsta.2018.0207
About the Authors
D. P. ShvetsovRussian Federation
Daniil P. Shvetsov, Engineer of the Research Center of Physics of Metallic Liquids, Ural Federal University named after the first President of Russia B.N. Yeltsin; Head of Technical Control Department, LLC “Precision Casting Center”
19 Mira Str., Yekaterinburg 620002, Russian Federation
18, bld. 4 Frontovykh Brigad Str., Yekaterinburg 620078, Russian Federation
O. A. Chikova
Russian Federation
Ol’ga A. Chikova, Dr. Sci. (Phys.–Math.), Prof. of the Chair of Physics
19 Mira Str., Yekaterinburg 620002, Russian Federation
V. S. Tsepelev
Russian Federation
Vladimir S. Tsepelev, Dr. Sci. (Eng.), Prof., Director of the Research Center of Physics of Metallic Liquids
19 Mira Str., Yekaterinburg 620002, Russian Federation
N. I. Sinitsin
Russian Federation
Nikolai I. Sinitsin, Cand. Sci. (Phys.–Math.), Assist. Prof. of the Chair of Physics, Senior Researcher of the Research Center of Physics of Metallic Liquids
19 Mira Str., Yekaterinburg 620002, Russian Federation
V. V. V’yukhin
Russian Federation
Vladimir V. V’yukhin, Research Associate of the Research Center of Physics of Metallic Liquids
19 Mira Str., Yekaterinburg 620002, Russian Federation
Review
For citations:
Shvetsov D.P., Chikova O.A., Tsepelev V.S., Sinitsin N.I., V’yukhin V.V. Physico-chemical analysis of 12Kh18N9TL steel melts for quality control of cast products. Izvestiya. Ferrous Metallurgy. 2025;68(5):534-542. https://doi.org/10.17073/0368-0797-2025-5-534-542