Scroll to:
Influence of amphoteric oxides content on valve effect characteristics of electric arc
https://doi.org/10.17073/0368-0797-2025-5-526-533
Abstract
The paper presents the experimental data on the effect of amphoteric oxides content (Al2O3 and Fe2O3 ) in slags on the metallurgical slags properties. It is noted that the parameters of the electric arc valve effect, such as the constant component of the arc voltage or the constant component of the electrode current, can act as a criterion for assessing the slag basicity. Up to a slag content of 18 wt. %, aluminum oxide exhibits mainly basic properties, and above that, acidic properties. For Fe2O3 , the threshold value is the content of 20 wt. %. The data obtained make it possible to more reasonably adjust the smelting slag mode. In particular, for the current trend on metallurgical enterprises to replace fluorspar in the out-of-furnace steel processing with other slag liquefiers, these data allow to determine the limit of aluminum oxide content at which the conditions of metal refining from sulfur will not be degraded. For arc steelmaking furnaces, this technique is one of the options for non-contact operational assessment of the metal bath state, the foaming slag quality to cover the arcs, and the metal oxidation degree at the melting end and its readiness for tapping. The use of constant components of the arc voltage and current in the electrode for operational control of the tendency of amphoteric oxides to basic or acidic properties during melting in industrial conditions is not possible due to a large number and multidirectional influence of the slag components. Nevertheless, this technique will be useful in control of steelmaking technological process with digital twin models and their accompanying databases.
Keywords
For citations:
Sivtsov A.V., Sheshukov O.Yu., Egiazaryan D.K., Tsymbalist M.M., Orlov P.P. Influence of amphoteric oxides content on valve effect characteristics of electric arc. Izvestiya. Ferrous Metallurgy. 2025;68(5):526-533. https://doi.org/10.17073/0368-0797-2025-5-526-533
Introduction
Improving steel quality is one of the key objectives of electric steelmaking. The parameters of the slag mode – composition, viscosity, quantity, and rate of slag formation – have a significant effect on metal quality, process yield, refractory lining durability, and other technological factors. This issue becomes particularly relevant given the continuous decline in scrap quality and the corresponding increase in harmful impurities, most of which are sulfur and phosphorus. Low-quality charge materials lead to higher energy and material losses and cause a considerable decrease in the technical and economic performance indicators of the smelt. In particular, melting time, energy consumption, and the use of ferroalloys and slag-forming materials increase, while furnace productivity decreases.
Modern steelmaking technologies involve the use of electric arc furnaces (EAFs) and basic oxygen converters (BOFs) as the main units for producing unalloyed, non-deoxidized iron–carbon melts using scrap, pig iron, and metallized feedstock as charge materials [1 – 3]. The iron–carbon melt is transferred in ladles to secondary metallurgy units – ladle furnaces (LF) and vacuum treatment units – where the melt composition is adjusted to meet the requirements of specific steel grades and operations are carried out to improve steel quality by removing oxygen, sulfur, and nonmetallic inclusions1 [4; 5]. Without secondary metallurgy units, a resource- and energy-efficient technology for liquid steel production is not feasible.
Steel production in EAFs accounts for a significant share of global output, second only to the BOF process. This process is associated with high energy consumption, which makes energy and technological optimization of great importance. However, optimization efforts often focus not on technological aspects but on the cost of the main resource – electric power [6] – or on auxiliary operations that are not directly related to melting [7].
Unlike BOF, EAF is a unit with a large number of adjustable parameters and process control tools that can operate directly within the working zone – even during arcing operation. However, due to the presence of molten metal and slag, burning arcs, and high currents, the working zone is highly aggressive and difficult to access for direct measurements and process control. Therefore, mathematical modeling plays a major role in effective process control [8 – 10], both in general-purpose studies and when accounting for the specific features of a particular unit – for example, when using hot-briquetted iron or various off-gas post-combustion modes [11; 12]. On the other hand, the measurement of EAF electrical characteristics, which eliminates the difficulties associated with the aggressive working environment, faces problems of signal filtering and processing of the recorded signals under circuit-break events, short circuits, short-arc and long-arc operation, and changes in the chemical composition of the arc plasma [13].
Steelmaking slags are mainly composed of calcium and silicon oxides, which together typically account for 85 – 90 wt. % of the total oxide content. Their ratio (CaO/SiO2 ) is used as a proxy measure of an important technological parameter – the slag basicity. A more accurate assessment accounts for the contents of other acidic and basic oxides, such as those of iron, magnesium, and manganese.
At the same time, the oxides of many metals – particularly aluminum, titanium, vanadium, iron, and chromium – are amphoteric, meaning that at certain contents they can exhibit either acidic or basic behavior. These oxides have been extensively studied in blast furnace ironmaking [14]. In blast furnaces, any adjustments to ironmaking process or charging process are associated with significant process dead time, i.e., a delayed process response to control interventions. Therefore, researchers model blast furnace slags and investigate the effects of individual components – including aluminum and titanium oxides – on slag viscosity, structure, and refining properties [15]. These oxides can have highly diverse effects: formation of nitrides and refractory spinels in high-temperature zones can extend campaign life, whereas their formation in other zones can decrease the effective working volume and disrupt furnace operation. At the same time, increasingly comprehensive theories are being developed to describe the behavior of amphoteric oxides based on slag structure data [16 – 18].
Interest in amphoteric oxides is driven by the fact that many metallurgical plants, seeking substitutes for fluorspar – a traditional slag liquefier – have begun to use alumina-containing materials composed primarily of aluminum oxides. Consequently, in out-of-furnace slags, the content of this oxide can reach 40 wt. %. It serves not only as a slag liquefier but also stabilizes the solidified slag, preventing its self-disintegration during cooling. However, the primary goal of out-of-furnace metal processing is sulfur removal, the rate and extent of which are determined by the refining ability of the slag.
To absorb substantial amounts of sulfur, the slag must be highly basic. Because basic oxides donate free oxygen anions that mediate sulfur transfer from metal to slag, their content must exceed that of acidic oxides. Yet, the behavior of amphoteric oxides – able to act as either donors or acceptors of these anions – remains difficult to predict. Accordingly, determining the range of aluminum oxide contents in refining slags that does not impede desulfurization – where Al2O3 acts predominantly as a donor of free oxygen anions – represents the second reason for the interest in amphoteric oxides.
The third reason concerns assessing the applicability of this approach to the smelting of alloyed steel grades, in which components forming amphoteric oxides may be present in considerable amounts in both the slag and the metal. During arcing, partial evaporation of components from the metal–slag melt surface occurs; these components enter the ionized gaseous phase forming the arc column, which may affect the electrical conductivity of the arc gap in different ways. This aspect is a subject of further research and is not considered in the present study.
Methods
Numerous studies in the scientific literature are based on experimental investigations of the influence of slag composition on the magnitude of the constant component of arc voltage (CCAV) [19 – 21]. In AC circuits with an electric arc this component most often arises due to the difference in electron work function between materials of different nature. Thus, in EAF, the work function of electrons from graphite exceeds that from iron, and as a result, a positively directed constant component of arc voltage appears across the arc gap. This phenomenon is known as the electric arc valve effect.
The current density of thermionic emission is described by the Richardson–Dushman equation
| \[j = A{T^2}\exp \left( { - \frac{{{\varphi _e}}}{{kT}}} \right),\] | (1) |
where А is the Richardson constant; Т is the temperature; φe is the electron work function; k is the Boltzmann constant.
The current emission density is strongly affected by both temperature and the chemical factor – the electron work function. While the current emission density from the graphite electrode remains nearly constant, for the second electrode – the molten material – this parameter depends significantly on the chemical composition of the melt. Therefore, the difference between the emission currents, which determines the CCAV magnitude, is governed by this composition.
There are data indicating a close relationship between CCAV and the oxygen content in the metal melt of an EAF during the refining stage [22]. In many respects, this relationship resembles the dependence of the electrode potential in an electrolytic cell on oxygen activity (ionic concentration), as described by the Nernst equation (2). This relationship underlies the operation principle of Celox sensors used in industrial practice to measure the oxidation potential of metal and slag:
| \[E = {E_0} + \frac{{RT}}{{nF}}\ln \left( {\frac{{{a_{{\rm{ox}}}}}}{{{a_{{\rm{red}}}}}}} \right),\] | (2) |
where E is the electrode potential; E0 is the standard electrode potential; R is the universal gas constant; T is the absolute temperature; n is the number of electrons involved in the process; F is the Faraday constant; aox and ared are the activities of the oxidizing and reducing agents, respectively.
To process the initial electrical signals, Fourier series decomposition was used, and the CCAV was determined as the zero term of the series. However, for the analysis of experimental data, not the CCAV but the constant component of electrode current (CCEC) was used, since its determination does not require any additional transformation of the initial voltage signal. For correct determination of the CCAV, the voltage drop across the electrode itself and the current leads must be subtracted from the constant component (CC) of the measured voltage signal. The authors have previously developed a procedure for calculating this voltage drop; however, a simpler approach involves determining the constant component of current from the derivative of the electrode current signal, obtained using a Rogowski coil [23].
The key technological parameters of steelmaking in the electric arc furnace – the slag basicity and the metal oxidation degree – are closely related to the CCAV. An increase in slag basicity (i.e., the predominance of basic oxides over acidic oxides) causes a decrease in the CCAV, whereas a rise in oxygen activity (its content) in the metal melt leads to its increase. These relationships were previously observed by the authors in laboratory EAF experiments.
The experimental setup was a single-phase arc furnace lined with magnesia and equipped with an observation window at the level of the arc gap. Electric power was supplied from a welding transformer through two graphite electrodes 30 mm in diameter. The transformer’s input voltage was 380 V, and the open-circuit voltage on the secondary side was 80 V.
A graphite crucible containing the material to be melted was placed on the lower electrode. The arc length was controlled by micrometer screws. Three electrical signals were recorded during the process: voltage across the electrodes u(t), arc current i(t), and the current derivative di/dt. The voltage was scaled to a level suitable for analog-to-digital conversion using a voltage divider. The current signal was measured as the voltage drop across a calibrated 0.3 Ω resistor installed in the secondary circuit of the transformer. The voltage proportional to the current derivative was taken from the output of the Rogowski coil. These analog signals were digitized at a sampling frequency of 100 kHz using a 12-bit Advantech PCI-1713 analog-to-digital converter (ADC).
Results and discussion
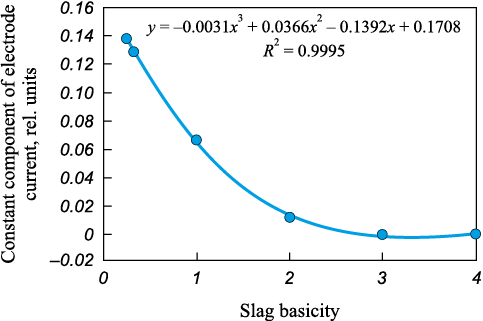
As an example, previously obtained results are presented for experiments investigating the influence of slag basicity (CaO/SiO2 ) on the parameters of the electric arc valve effect, particularly on the constant component of electrode current (Fig. 1). The experiments were performed as follows: the metallic sample was melted, and a solidified binary fused slag containing CaO and SiO2 at the specified ratio was added to the surface of the molten metal. After each addition, sufficient time was allowed for the slag to melt, dissolve, and reach equilibrium. The experiments were conducted separately for slags with basicity ratios of 0.25, 0.33, 1, 2, 3, and 4. The results confirmed that an increase in slag basicity leads to a decrease in the constant component of electrode current.
Fig. 1. Constant components of the electrode current |
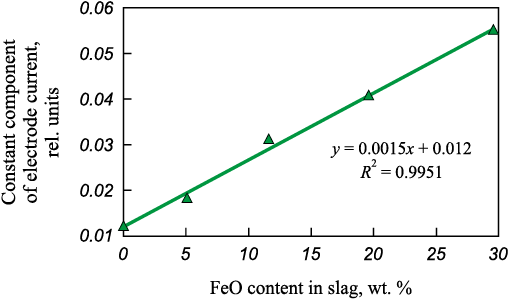
Considering this finding, an additional experiment was performed to evaluate the effect of the acidic oxide content in slag on its basicity and on the characteristics of the arc valve effect. A steel sample weighing 161 g was melted, and a slag consisting of dicalcium silicate with a 5 wt. % FeO addition was introduced onto the melt surface. After melting the metal, three successive portions of slag were charged into the furnace, totaling 51 g. Following the addition and dissolution of the slag, further FeO portions were added in amounts of 6.5, 8, and 10 g, respectively. The experimental results are shown in Fig. 2. It is evident that additions of an acidic oxide decrease slag basicity and, accordingly, increase its oxidizing capacity and the CCEC.
Fig. 2. Dependence of the electrode current constant component |
In general, the amphoteric properties of metal oxides are determined by their ability to accept or donate valence electrons [24]. It is therefore reasonable to assume that additions of Al2O3 and Fe2O3 to the slag affect its chemical properties and the parameters of the electric arc valve effect depending on their content in the slag. Consequently, one may expect nonmonotonic behavior in the dependence of the CCEC.
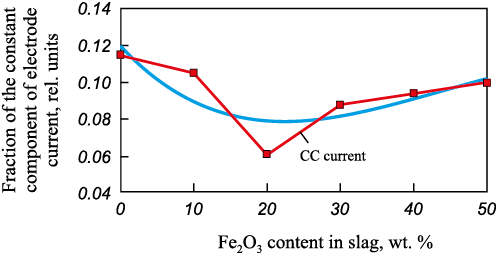
Experiments to assess the effect of Fe2O3 content on slag properties were carried out using a similar procedure. As shown in Fig. 3, a minimum in the CCEC was observed at an Fe2O3 concentration of about 20 wt. %. Thus, up to 20 wt. %, Fe2O3 behaves as a basic oxide, promoting an increase in slag basicity, whereas at contents above 20 wt. %, it exhibits acidic properties, reducing basicity. In practice, it is not possible to determine the individual contents of iron oxides (FeO, Fe2O3 , or Fe3O4 ) in slag. It is also unlikely that the proposed method of assessing them via the constant component of voltage or current would provide sufficient accuracy. Nevertheless, experimental studies in this direction can be extremely useful for addressing general tasks in the control and optimization of steelmaking technological processes in electric arc furnaces.
Fig. 3. Dependence of the electrode current constant component |
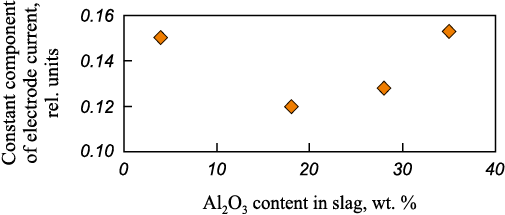
Among metal oxides, aluminum oxide most clearly exhibits amphoteric behavior. Experiments similar to those described above were conducted using the laboratory arc furnace. According to the data presented in Fig. 4, additions of Al2O3 up to about 18 wt. % lead to a decrease in the CCEC, whereas at contents above 20 wt. %, it increases, indicating the predominance of acidic properties of this oxide. The approximate Al2O3 content at which a sharp transition from basic to acidic behavior occurs is about 30 wt. %. Therefore, increasing the Al2O3 content in refining slags up to 18 wt. % has a beneficial effect on arc stability and on refining properties such as viscosity, interfacial tension, and desulfurizing ability.
Fig. 4. Dependence of the electrode current constant component |
From a practical standpoint, it should be noted that Al2O3 additions below 20 wt. % do not provide mechanical stability to refining slags without the use of additional stabilizing components, making them prone to spontaneous disintegration into fine powder. Increasing the Al2O3 content from 18 to 30 wt. % has only a minor adverse effect on arc operation but allows the stabilization of refining slags without additional methods. However, introducing larger amounts of Al2O3 deteriorates not only the refining characteristics of the slag but also its electrical parameters, adversely affecting both the arc stability and the electrical characteristics of the melting process.
Conclusions
Laboratory studies of the effects of Fe2O3 and Al2O3 contents in slag on the CCEC showed that these oxides exhibit basic behavior at contents below 20 and 18 wt. %, respectively, and acidic behavior at higher contents. This provides a stronger basis for defining the limiting aluminum oxide content in refining slags, considering both refining performance and slag stabilization.
Further investigation of other amphoteric oxides and their impact on arc characteristics (CCEC and CCAV) using the proposed approach may face several challenges. Metals forming these oxides tend to undergo reduction (chromium, vanadium), carbide formation (chromium, titanium), or nitride formation (chromium, vanadium, titanium). In addition, the laboratory tests used graphite crucibles, which, for other oxides, can substantially affect the slag phase composition, the accuracy of recorded signals, and the reliability of their interpretation.
Using the CCAV and CCEC for real-time control of an oxide’s tendency toward basic or acidic behavior during industrial heats is not feasible because of the large number of slag constituents and their complex, often opposing effects. Even so, the method is well suited for process control in digital twin models [25 – 27] and in associated databases. In particular, given the current trend at metallurgical plants to replace fluorspar with other slag liquefiers during out-of-furnace steel processing, these data help define the upper aluminum oxide content that does not impair sulfur removal from metal. For electric arc furnaces, the method also offers a non-contact, real-time means of assessing the metal bath condition, the quality of slag foaming for arc shielding, and the metal oxidation degree at the end of melting and the heat’s readiness for tapping.
References
1. Bigeev A.M., Bigeev V.A. Metallurgy of Steel. Magnitogorsk: MSTU; 2000:544. (In Russ.).
2. Gladkikh V.A., Gasik M.I., Ovcharuk A.N., Prokhodak Yu.S. Design and Equipment of Electric Steelmaking and Ferroalloy Workshops: Textbook. Dnepropetrovsk: Sistemnye tekhnologii; 2004:736. (In Russ.).
3. Bornatskii I.I., Mikhnevich V.F., Yargin S.A. Steelmaking. Moscow: Metallurgiya; 1991:400. (In Russ.).
4. Dyudkin D.A., Bat’ S.Yu., Grinberg S.E., Marintsev S.N. Steel Production on a Ladle-Furnace Unit. Donetsk: Yugo-Vostok, LTD; 2003:300. (In Russ.).
5. Povolotskii D.Ya., Gudim Yu.A., Zinurov I.Yu. Design and Operation of Heavy-Duty Arc Steelmaking Furnaces. Moscow: Metallurgiya; 1990:176. (In Russ.).
6. Shyamal S., Swartz C.L.E. Real-time energy management for electric arc furnace operation. Journal of Process Control. 2019;74: 50–62. https://doi.org/10.1016/j.jprocont.2018.03.002
7. Logunova O.S., Oshurkov V.A., Pavlov V.V. The method of efficiency definition of new functional task in electric arc furnace control system. IOP Conference Series: Materials Science and Engineering. 2020;718:012011. https://doi.org/10.1088/1757-899X/718/1/012011
8. Abadi M.M., Tang H., Rashidi M.M. A review of simulation and numerical modeling of electric arc furnace (EAF) and its processes. Heliyon. 2024;10(11):e32157. https://doi.org/10.1016/j.heliyon.2024.e32157
9. Kozyra J., Lozynskyy A., Łukasik Z., Kuśmińska-Fijałkowska A., Kutsyk A., Kasha L. Increasing the level of autonomy of control of the electric arc furnace by weakening interphase interactions. Energies. 2023;16(24):8114. https://doi.org/10.3390/en16248114
10. Saboohi Y., Fathi A., Škrjanc I., Logar V. Comprehensive electric arc furnace model for simulation purposes and model-based control. Steel Research International. 2017;88(3): 1600083. https://doi.org/10.1002/srin.201600083
11. Irawan A., Kurniawan T., Alwan H., Muslim Z.A., Akhmal H., Firdaus M.A., Bindar Y. An energy optimization study of the electric arc furnace from the steelmaking process with hot metal charging. Heliyon. 2022;8(11):e11448. https://doi.org/10.1016/j.heliyon.2022.e11448
12. Ugarte O., Li J., Haeberle J., Frasz T., Okosun T., Zhou C.Q. CFD modeling of HBI/scrap melting in industrial EAF and the impact of charge layering on melting performance. Materials. 2024;17(21):5139. https://doi.org/10.3390/ma17215139
13. Wang B., Mao Z. Detecting outliers in electric arc furnace under the condition of unlabeled, imbalanced, non-stationary and noisy data. Measurement and Control. 2018;51(3-4): 83–93. https://doi.org/10.1177/0020294018771097
14. Dmitriev A.N., Chen’ K., Zolotykh M.O., Vit’kina G.Yu. Mathematical Modeling of Blast-Furnace Process. Yekaterinburg: Publishing House AMB; 2023:232. (In Russ.).
15. Zhou W., Li T., Lan D., Sun C., Yang S. Influence of TiO2 , Al2O3 , and basicity on viscosity and structure of high titanium-bearing blast furnace slag. Materials. 2023;16(7):2575. https://doi.org/10.3390/ma16072575
16. Bi Z., Li K., Jiang C., Zhang J., Ma S., Alberto C., Sun M., Bu Y., Barati M., Ren S. New insights into the traditional charge compensation theory: Amphoteric behavior of TiO2 under the guidance of supply-demand relationship. ACS Omega. 2022;7(24):21225–21232. https://doi.org/10.1021/acsomega.2c02252
17. Zhou C., Li J., Wang S., Zhao J., Ai L., Chen Q., Chen Q., Zhao D. Development of molecular dynamics and research progress in the study of slag. Materials. 2023;16(15):5373. https://doi.org/10.3390/ma16155373
18. Jiang C., Li K., Bi Z., Ma S., Zhang J., Liu B., Li J. Developments in atomistic and nano structure evolution mechanisms of molten slag using atomistic simulation methods. Nanomaterials. 2024;14(5):464. https://doi.org/10.3390/nano14050464
19. Pedro A.A., Arlievskii M.P., Kurtenkov R.V. The constant component of the phase voltage during melting of zirconium electrocorundum. Elektrometallurgiya. 2011;(7):37–39. (In Russ.).
20. Pedro A.A., Suslov A.P. Valve effect in an electrode furnace. Tsvetnye Metally. 2012;(12):91–95. (In Russ.).
21. Sivtsov A.V., Sheshukov O.Yu., Tsymbalist M.M., Nekrasov I.V., Egiazar’yan D.K. The valve effect of an electric arc and problems in controlling electric-arc furnaces. Metallurgist. 2015;59(5-6):380–385. https://doi.org/10.1007/s11015-015-0113-6
22. Sivtsov A.V., Sheshukov O.Yu., Nekrasov I.V., Tsymbalist M.M., Egiazaryan D.K., Orlov P.P. On some features of use of constant arc voltage component for control of metal oxidation at stage of steel refinement. Elektrometallurgiya. 2020;(1):2–8. (In Russ.).
23. Sivtsov A.V., Tsymbalist M.M., Sheshukov O.Yu., Nekrasov I.V., Egiazar’yan D.K. Dynamic volt-ampere characteristics of electric arc circuits as a means of monitoring and controlling technological modes of arc steelmaking furnaces. In: Modern Problems of Electrometallurgy of Steel. Proceedings of the XVI Int. Conf.: in 2 Parts. 2015:144–149. (In Russ.).
24. Povolotskii D.Ya. Physico-Chemical Fundamentals of Steelmaking Processes. 2nd ed. Chelyabinsk: SUSU; 2007:183. (In Russ.).
25. Reuter M.A., Kaußen F., Geimer S., Borowski N., Degel R., Lux T. Metallurgical slags enable the circular economy – digital twins of metallurgical systems. World of Metallurgy – ERZMETALL. 2021;74(4):192–202.
26. Rauch L., Pietrzyk M. Digital twins as a modern approach to design of industrial processes. Journal of Machine Engineering. 2019;19(1):86–97. https://doi.org/10.5604/01.3001.0013.0456
27. Xiang F., Zhi Z., Jiang G. Digital twins technology and its data fusion in iron and steel product life cycle. In: 2018 IEEE 15th Int. Conf. on Networking, Sensing and Control (ICNSC). Zhuhai, China. 2018. https://doi.org/10.1109/ICNSC.2018.8361293
About the Authors
A. V. SivtsovRussian Federation
Andrei V. Sivtsov, Dr. Sci. (Eng.), Leading Researcher of the Laboratory of Technogenic Formations Problems
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
O. Yu. Sheshukov
Russian Federation
Oleg Yu. Sheshukov, Chief Researcher of the Laboratory of Powder, Composite and Nano-Materials, Vatolin Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences; Dr. Sci. (Eng.), Prof., Director of the Institute of New Materials and Technologies, Ural Federal University named after the first President of Russia B.N. Yeltsin
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
19 Mira Str., Yekaterinburg 620002, Russian Federation
D. K. Egiazaryan
Russian Federation
Denis K. Egiazaryan, Cand. Sci. (Eng.), Senior Researcher, Head of the Laboratory of Technogenic Formations Problems, Vatolin Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences; Assist. Prof. of the Chair of Metallurgy of Iron and Alloys, Ural Federal University named after the first President of Russia B.N. Yeltsin
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
19 Mira Str., Yekaterinburg 620002, Russian Federation
M. M. Tsymbalist
Russian Federation
Mikhail M. Tsymbalist, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Reduction Processes
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
P. P. Orlov
Russian Federation
Pavel P. Orlov, Cand. Sci. (Eng.), Engineer, Senior Lecturer of the Chair of Metallurgy of Iron and Alloys of the Institute of New Materials and Technologies
19 Mira Str., Yekaterinburg 620002, Russian Federation
Review
For citations:
Sivtsov A.V., Sheshukov O.Yu., Egiazaryan D.K., Tsymbalist M.M., Orlov P.P. Influence of amphoteric oxides content on valve effect characteristics of electric arc. Izvestiya. Ferrous Metallurgy. 2025;68(5):526-533. https://doi.org/10.17073/0368-0797-2025-5-526-533