Scroll to:
Formation of iron-carbon melts and technological aspects of carburization
https://doi.org/10.17073/0368-0797-2025-5-506-516
Abstract
Based on the results of viscometric studies of the patterns of precision iron-carbon melts formation, the need to take into account the structural state of the initial iron melt and the carbon material when developing the optimal carburization technology is substantiated. Considering the kinematic viscosity value and stability of the values of this structure-sensitive property as indicators of the non-equilibrium degree of the iron-carbon melt structural state, it is shown that the optimal solution should be considered to be the carburization of liquid iron with a predominantly fcc-like short-range order structure, which can be facilitated by introducing carbon into the charge composition, forced heating and melting with the formation of a melt with significant overheating above the liquidus temperature. Using the data of X-ray structural analysis, it was experimentally established that when using carbon materials subjected to graphitization for carburization, it is advisable to reduce the proportion of the amorphous phase, increase the size of crystallites and the graphitization degree, and improve the structural homogeneity. The experimentally determined difference in the nature of melt formation during carburization of pure iron and in the case of simultaneous deoxidation and carburization of highly oxidized metal is associated with the process of deactivation of the carbon material surface by oxygen, the development degree of which depends on reactivity of the carbon material and decreases with an increase in the degree of its graphitization and a decrease in the defectiveness of the crystal structure. During the simultaneous deoxidation and carburization of highly oxidized iron, a significant role of the deoxidizer type was established, which is associated with the different partial influence of the materials used on structural state of the iron melt and with the different neutralization degree of the effect of oxygen on deactivation of the surface of carburizer particles.
Keywords
For citations:
Gudov A.G., Burmasov S.P., Smirnov L.A. Formation of iron-carbon melts and technological aspects of carburization. Izvestiya. Ferrous Metallurgy. 2025;68(5):506-516. https://doi.org/10.17073/0368-0797-2025-5-506-516
Introduction
Carburization has become an integral element in steel production using modern high-intensity technologies. This increases the relevance of studying the patterns of iron–carbon melt formation, since the establishment of an equilibrium structural state of the melt prior to crystallization is a prerequisite for achieving the maximum level of service properties of the metal [1 – 4]. Different degrees of completion of the relaxation process of the non-equilibrium structural state of the melt caused by carburization lead to an increase in the heterogeneity of the metal and the instability of its properties [5; 6]. Insufficient attention to this factor may explain why the use of modern technological schemes for steel production, despite their enhanced ability to refine the metal from undesirable impurities, does not guarantee the stability of high service properties of steel products [7].
Modern methods for assessing the degree of non-equilibrium of the structural state of iron melts are based on the study of structure-sensitive properties of melts, in particular on viscometric studies. In most cases, a higher degree of equilibrium of the melt’s structural state at a fixed composition corresponds to higher kinematic viscosity values and greater stability of this structure-sensitive property [1 – 4].
As shown in the authors’ previous studies [8], iron melts can exist within a spectrum of similar structural states, and the stability of one of these states increases when a solution with carbon is formed. To further elucidate the regularities of iron carburization, the authors continued viscometric studies on the formation of precision iron–carbon melts. The experiments were carried out in a vacuum high-temperature viscometer under an atmosphere of ultra-pure helium (>99.9999 %), using the torsional oscillation method of a crucible containing the metal to determine the kinematic viscosity of the melt. This method minimizes the perturbing influence on the structural state of the melt. The results obtained at this stage make it possible to identify a number of factors that must be considered when developing the parameters of the optimal carburization technology for iron.
Influence of structural state of the initial iron melt
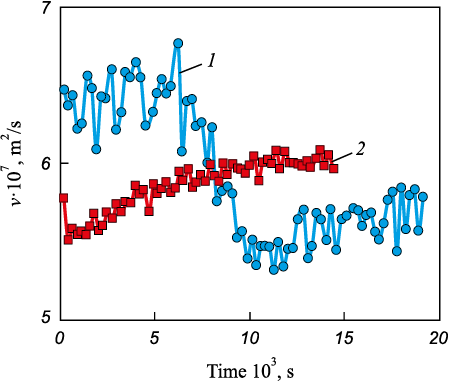
Fig. 1 presents the results of a viscometric study of the formation of a pure iron melt at 1873 K obtained through a multistage hydrogen refining process of carbonyl iron. Two heating modes up to 1873 K were used:
Fig. 1. Results of the viscometric study of dynamics of pure iron melt formation |
– mode 1: isothermal holding for 900 s at 1473 K (within the thermodynamic stability region of γ-Fe), followed by forced heating to 1873 K within 120 s;
– mode 2: holding for 1200 s near the melting point (within the thermodynamic stability region of δ-Fe), nearly equilibrium slow melting for 8400 s with minimal overheating above the melting temperature, and subsequent heating to 1873 K at an average rate of 2 K/min.
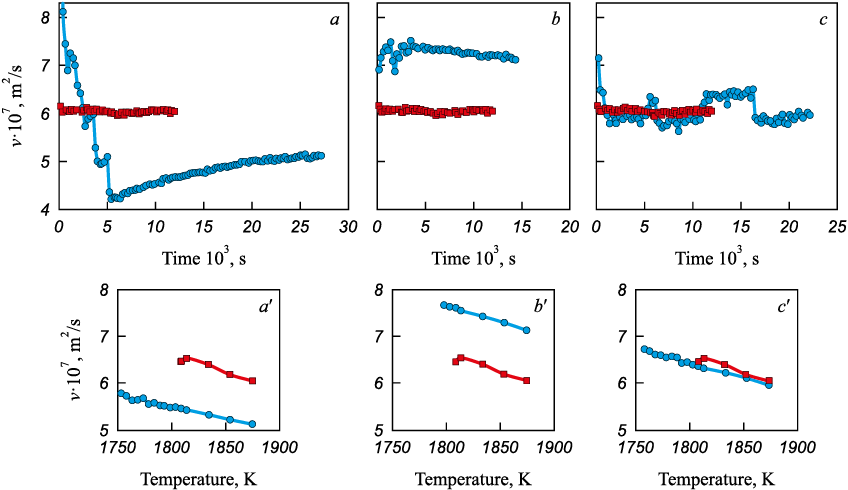
The obtained data indicate a significant influence of the heating and melting mode on the structural state of the resulting iron melt. According to the authors, the difference in the structural states of the melt may be associated with the “inheritance” of different short-range order structures from the initial solid metal. In the first case, the increased kinematic viscosity values after melting can be attributed to a more densely packed structure with a predominantly FCC-like atomic coordination. In the second case, a BCC-like configuration of atomic micro-groupings is inherited. It is worth noting that, under the thermodynamic conditions implemented for pure iron melts, the structural state with a predominantly FCC-like atomic coordination is relatively unstable. During isothermal holding at 1873 K, a transition occurs to a structural state with predominantly BCC-like atomic coordination. The pronounced high-frequency oscillations of kinematic viscosity observed in the case of forced heating may be attributed to significant micro-inhomogeneity of the forming structural state of the melt, caused by partial transformation of γ-Fe to δ-Fe as a result of the overlap of two phase transitions and the coexistence of FCC- and BCC-like atomic groupings.
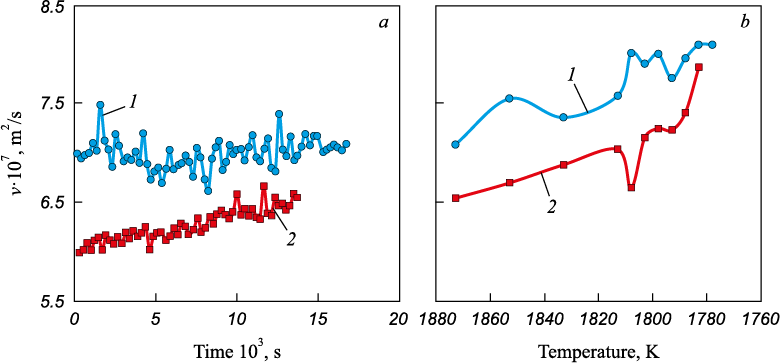
The implementation of carburization under the above heating and melting modes can therefore be associated with the formation of a carbon solution in different structural states of the initial iron melt. As shown in Fig. 2, a, the formation of an iron–carbon melt under the first heating and melting mode leads to the development of a structural state with a markedly higher kinematic viscosity, which, according to currently accepted views, corresponds to a more equilibrium structural state of the melt. This is further supported by an analysis of the stability of the realized structural states of the iron–carbon melt: for the state with lower kinematic viscosity, there is a clear tendency to transform into a state with higher viscosity, both under isothermal holding at 1873 K and during subsequent cooling from 1873 K to crystallization (Fig. 2, b).
Fig. 2. Results of the viscometric study of dynamics of the Fe – 0.1 % C melt formation |
The above results suggest that, during carburization of iron, the optimal process should be considered the carburization of liquid iron possessing a predominantly FCC-like short-range order structure. However, it is also possible that the instability and non-equilibrium character of the initial structural state – enhanced by the strongly non-equilibrium melting mode – play a substantial, if not decisive, role in this process.
Influence of the carbon material structure
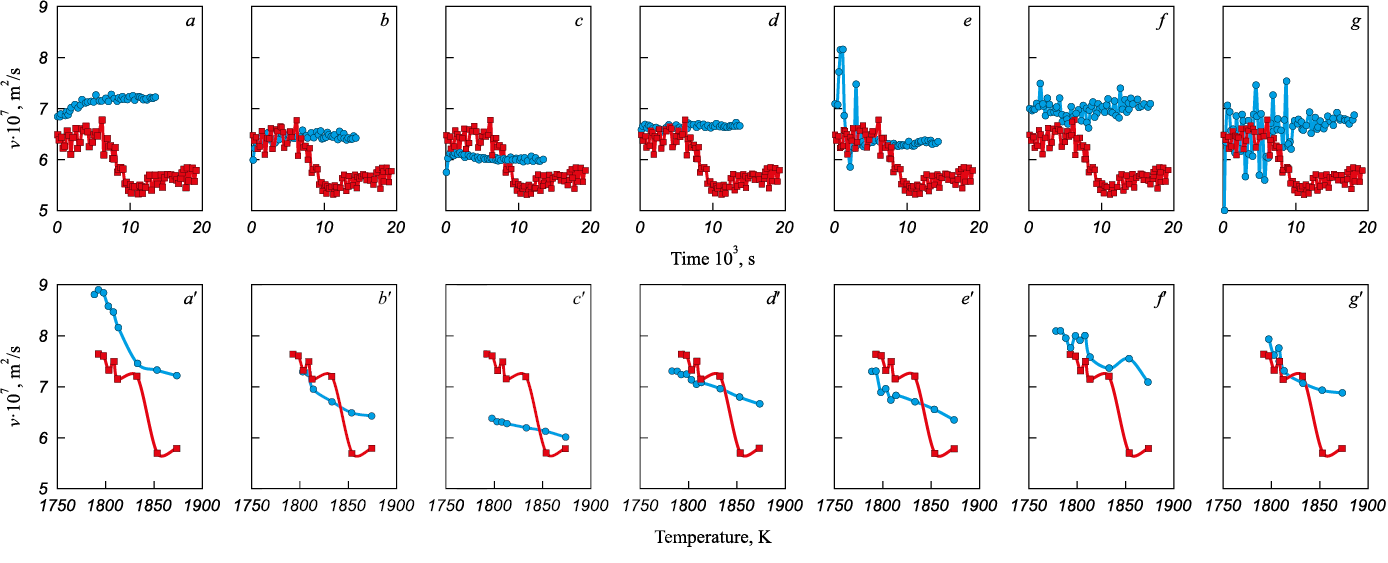
From the standpoint of differences in carburizer quality, current research mainly focuses on its chemical composition – primarily the carbon content (or ash content) and nitrogen concentration. According to the authors, attention should also be paid to the structure of the carbon material itself. It is the structure, above all, that may determine the influence of the carbon material type on the nature of melt-formation behavior and the resulting structural state after iron carburization (Fig. 3).
Fig. 3. Results of the viscometric study of dynamics of the Fe – 0.1 % C melt formation during isothermal holding at 1873 K and changes during subsequent cooling to crystallization in comparison |
Based on X-ray diffraction (XRD) analysis (Table 1), comparing carbon materials subjected to graphitization (graphites of different grades, e.g., GMZ and GII-A, and electrode scrap), the data indicate that a decrease in the kinematic viscosity of the resulting iron–carbon melt – and, in line with current understanding, a corresponding increase in the degree of structural non-equilibrium – is associated with a higher amorphous-phase fraction (likely due to a non-graphitizing binder or its partial graphitization), a smaller crystallite (coherent domain) size, and a lower degree of graphitization (three-dimensional ordering within crystallites) [9].
Table 1. Results of X-ray structural analysis of carbon materials
|
The results shown in Fig. 4, obtained from carburization tests in which the same pure converter iron was carburized using graphite of GII-A grade supplied by three different manufacturers, also indicate that it is not the graphite type itself but its structure that plays the decisive role. Along with confirming the previously described effect of the graphite structural parameters (Table 2) on the viscosity level of the iron–carbon melt, these data demonstrate a substantial influence of the initial graphite structural heterogeneity on both the relaxation time of the melt’s structural-state non-equilibrium after graphite addition and the amplitude of the high-frequency oscillatory component of kinematic viscosity changes, which can be associated with the degree of micro-inhomogeneity of the melt. The maximum structural heterogeneity of graphite – characterized by a combination of a relatively low-defect crystalline phase and a large amorphous phase fraction (Fig. 4, b) – leads to the greatest micro-inhomogeneity and instability of the structural state of the iron–carbon melt. Reducing the graphite heterogeneity, either by increasing the defectiveness of the crystalline phase (Fig. 4, a) or by decreasing the amorphous phase fraction (Fig. 4, c), reduces both the amplitude of the high-frequency component and the stabilization time of kinematic viscosity values, although at different levels of this structure-sensitive property, corresponding to different degrees of non-equilibrium structural state of the melt. The behavior of the subsequent polytherms indicates a higher degree of structural non-equilibrium, characterized by lower kinematic viscosity values.
Fig. 4. Results of the viscometric study of dynamics of the melt formation during isothermal holding
Table 2. Results of X-ray structural analysis of graphite of GII-A grade
|
The mechanism of carbon transfer from the carburizer into liquid iron is one of the key aspects of the physicochemical nature of the carburization process. According to A.A. Vertman and A.M. Samarin [10], based on the relatively low activation energy of graphite dissolution and the specific features of its crystal structure, the dissolution of graphite in liquid iron proceeds in at least two stages: (1) detachment of flat graphite layers from the surface of the solid particle and (2) dissolution of these detached layers.
Accordingly, in addition to crystallite defectiveness and the character and degree of ordering of the carbon hexagonal layers, another significant factor is the type of bonding between the hexagonal networks and their stacks. For different graphites, researchers agree that the interlayer bonds – whether van der Waals, metallic, or weakened covalent [11] – are relatively weak yet stable. The low strength of the bonds between carbon hexagons (in the form of short hydrocarbon chains [12]) and their partial rupture upon heating facilitate the dissolution of carbon into pure iron when using anthracite, thereby promoting the formation of the most equilibrium structural state, as indicated by the highest kinematic viscosity values. In contrast, when glassy carbon is used, the strong and diverse bonds between hexagonal stacks – including oxygen bridges and triple conjugated (–C≡C–) or double cumulated (=C=C=) C–C bonds [9] – significantly hinder melt formation, increasing the micro-inhomogeneity and instability of its structural state. The even stronger bonding of carbon hexagons in carbon nanotubes may account for the greatest difficulty in melt formation when this material is used as a carburizer.
The use of another allotropic form of carbon – diamond – for carburization of pure iron promotes the formation of a melt structural state with relatively high kinematic viscosity. The noticeable oscillatory component of viscosity variation during melt formation also indicates its micro-inhomogeneity. This behavior may be explained by two factors. First, the spatial configuration of carbon atoms in diamond is similar to that in austenite, allowing carbon to enter the iron lattice as a complete diamond cluster, forming a hybrid of interstitial and substitutional solid solutions [13], which likely facilitates the formation of an fcc-like structural state of the melt. Second, when heated above 1273 K, diamond tends to transform spontaneously into graphite, forming at 1873 K an intermediate carbon structure – a crystalline hybrid of cubic (diamond) and hexagonal (graphite) lattices [14]. Therefore, the observed melt-formation behavior may result from the superposition of graphite dissolution and partial diamond-to-graphite transformation, which undoubtedly enhances the micro-inhomogeneity of the melt’s structural state.
Simultaneous carburization and deoxidation of metal
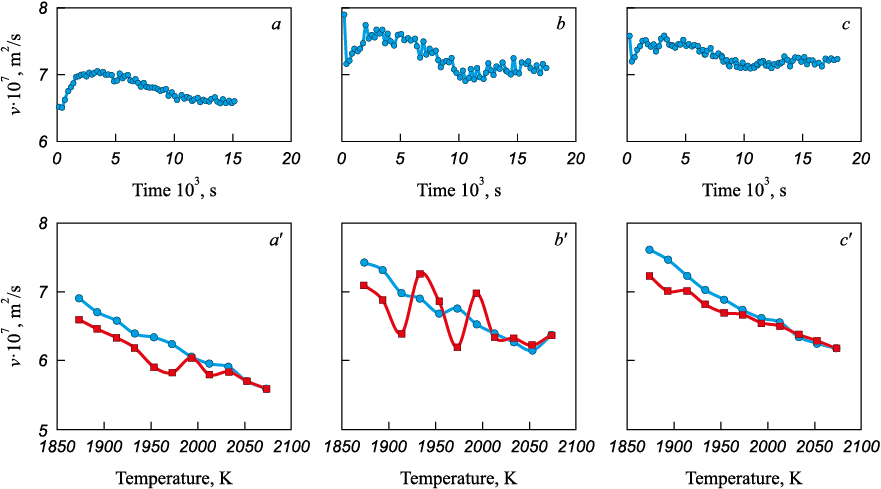
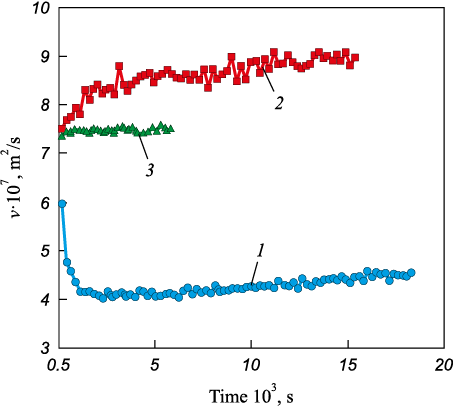
Previously, experiments demonstrated that dissolved oxygen prolongs the time required for the melt to reach structural equilibrium during iron carburization, providing the rationale for treating deoxidation and carburization as concurrent operations [15]. Accordingly, experiments were conducted on simultaneous deoxidation and carburization of iron (Fig. 5). Carbonyl iron with an oxidation level of 870 ppm was used as the base metal. Deoxidation was performed with aluminum to obtain a residual concentration of 0.03 %.
Fig. 5. Results of the viscometric study of dynamics of the Fe – 0.1 % C melt formation |
Analysis of the data shows that the previously described influence of the structural parameters of graphitized carbon materials on the degree of structural non-equilibrium of the melt generally persists under these conditions. In particular, using graphite of GMZ grade yields a more equilibrium structural state of the melt than using graphite of GII-A grade. At the same time, melt-formation behavior changes fundamentally when anthracite is used for carburization: the relaxation time becomes the longest, while the kinematic viscosity is the lowest. According to A.A. Vertman [16], dissolved oxygen increases the stability of carbon layer stacks by forming CxOy-type oxide films on their surfaces. Under the present conditions, this process is likely most pronounced for natural carbon materials such as anthracite. By contrast, with graphitized materials, oxygen-induced surface deactivation is mitigated because graphitization lowers reactivity and reduces crystal-structure defectiveness [14].
It should be noted separately that the established regularities are consistent with previously obtained experimental data under industrial conditions [3 – 5].
Type of deoxidizer
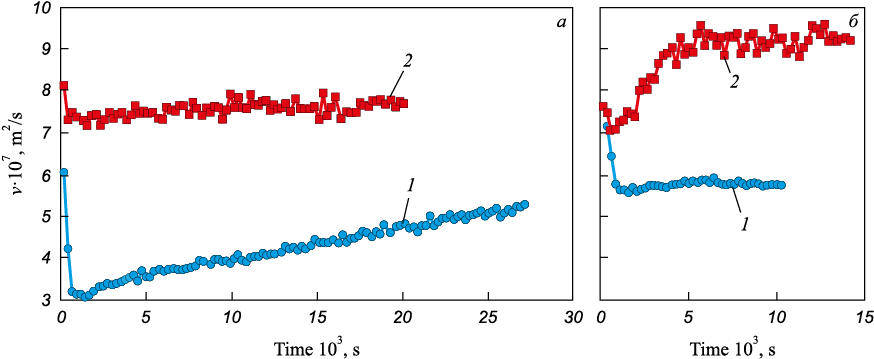
Under simultaneous deoxidation and carburization, the choice of deoxidizer – in addition to the carbon material – also affects melt-formation behavior. As shown in Fig. 6, deoxidizing carbonyl iron with aluminum and with calcium produces fundamentally different structural states of the melt. With aluminum, the melt shifts to a state with significantly lower kinematic viscosity than that of the starting carbonyl iron. In contrast, calcium deoxidation drives structural changes that increase kinematic viscosity. This response is consistent with a calcium-induced perturbation of the melt’s structural state that drives it toward a higher degree of structural equilibrium [17].
Fig. 6. Results of the viscometric study of dynamics of the melt formation during deoxidation |
Experimental results presented in Fig. 7 show that this deoxidizer-specific effect on the melt’s structural state persists under simultaneous deoxidation and carburization. Calcium yields a structural state with substantially higher kinematic viscosity than aluminum, indicating a more nearly equilibrium structure. Moreover, when anthracite is used for carburization, the relaxation time of the introduced non-equilibrium is considerably shorter. The established difference, apart from the distinct effects of the deoxidizers on the structural state of the iron melt, may also be related to the fact that calcium, as a stronger deoxidizer, more effectively neutralizes the influence of oxygen on the deactivation process of the carburizer particle surface.
Fig. 7. Results of the viscometric study of dynamics of the melt formation with simultaneous |
Overall, the findings show that, during simultaneous deoxidation and carburization, calcium favors the formation of an optimal melt structural state. Therefore, in choosing a deoxidizer, partial or full replacement of aluminum with calcium-containing agents (e.g., calcium carbide) should be considered, particularly for carbide treatment operations.
Conclusions
When forming an iron–carbon melt, the optimal route is carburization of liquid iron with a predominantly FCC-like short-range order. This can be achieved by introducing carbon into the charge, applying forced heating, and melting with substantial superheating above the liquidus.
The structural state of the melt during iron carburization is strongly governed by the structure of the carbon material. With graphitized carbons, it is advisable to reduce the amorphous-phase fraction, increase crystallite size and degree of graphitization, and improve structural homogeneity. Other significant factors include the type of bonding between hexagonal layers and their stacks and the allotropic form of carbon.
The choice of carburizer depends critically on the thermodynamic conditions of melt formation: for pure iron, anthracite gives the most favorable result, whereas for highly oxidized metal, graphitized materials are more effective.
Under simultaneous deoxidation and carburization, an optimal structural state of the iron–carbon melt is promoted by partially or fully substituting aluminum with calcium-containing agents, such as calcium carbide.
References
1. Baum B.A., Khasin G.A., Tyagunov G.V., etc. Liquid Steel. Moscow: Metallurgiya; 1984:208. (In Russ.).
2. Baum B.A., Tyagunov G.V., Baryshev E.E., Tsepelev V.S. Equilibrium and nonequilibrium states of metallic melts. In: Fundamental Research of Physical Chemistry of Metallic Melts: Proceedings. Moscow: IKTs Akademkniga; 2002:214–228. (In Russ.).
3. Burmasov S.P., Gudov A.G., Degai A.S., Stepanov A.I., Smirnov L.A. Formation of iron-based melts and scope for improvement in steel properties. Steel in Translation. 2010;40(8):741–745. https://doi.org/10.3103/S0967091210080127
4. Zuev M.V., Burmasov S.P., Stepanov A.I., Gudov A.G., Murzin A.V., Zhitlukhin E.G. Improvement in steel smelting by studying melt behavior. Steel in Translation. 2013;43(2):106–109. https://doi.org/10.3103/S0967091213020204
5. Gudov A.G., Burmasov S.P., Toporov V.A., Murzin A.V. Development of electric steelmaking technology based on the study of non-equilibrium of metal melts. In: Modern Problems of Steel Electrometallurgy. Proceedings of the XVIII Int. Conf., September 24-27, 2019, Yekaterinburg, Pervouralsk: in 2 parts. Part 1. Roshchin V.E. ed. Chelyabinsk: SUSU; 2019:3–25. (In Russ.).
6. Burmasov S.P., Gudov A.G., Zhitlukhin E.G., Parkhomenko I.P., Stolbov N.S. Directions for reducing chemical heterogeneity of continually cast pipe blank. Steel in Translation. 2023;53(9):785–791. https://doi.org/10.3103/s0967091223090048
7. Filip’ev S.N., Burmasov S.P., Gudov A.G., etc. Influence of semi-finished product production technology on the quality of liquid metal and the performance properties of spring steel. In: Proceedings of the XIII Int. Congress of Steelmakers, October 12–18, 2014, Polevskoi. Yekaterinburg: Ezaprint; 2014:82–87. (In Russ.).
8. Burmasov S.P., Gudov A.G., Smirnov L.A. Structural states of iron melt and its solutions with vanadium, silicon and carbon. Teoriya i tekhnologiya metallurgicheskogo proizvodstva. 2018;(1(24)):21–27. (In Russ.).
9. Fialkov A.S. Carbon-Graphite Materials. Moscow: Energiya; 1979:320. (In Russ.).
10. Vertman A.A., Samarin A.M. On the kinetics of carbon dissolution in liquid iron. Izvestiya AN SSSR. Metally. 1965;(1):46–54. (In Russ.).
11. Shulepov S.V. Physics of Carbon-Graphite Materials. Moscow: Metallurgiya; 1972:256. (In Russ.).
12. Kurakov Yu.I. Development of views on the structure of highly metamorphosed coals. Izvestiya vuzov. Severo-kavkazskii region. Estestvennye nauki. Prilozhenie. 2005;(6): 100–107. (In Russ.).
13. Kraposhin V.S. New mechanism of carbon dissolution in the austenite lattice during steel carburization and its behavior during martensitic and pearlitic transformations of austenite. In: Nauka i obrazovanie: Scientific Publication of Bauman Moscow State Technical University. 2011;(11):1–15. (In Russ.).
14. Ubbelohde A.R., Lewis F.A. Graphite and Its Crystal Compounds. London: Oxford University Press; 1960:217.
15. Zuev M.V., Burmasov S.P., Gudov A.G., Murzin A.V., Kuzyakin V.G. Carburization of crude steel from a superpowerful arc furnace. Steel in Translation. 2014;44(6):433–438. https://doi.org/10.3103/S0967091214060187
16. Vertman A.A. Microheterogeneity of metal melts and the problem of regulating the properties of castings. Fizika i khimiya obrabotki materialov. 1967;(3):132–141. (In Russ.).
17. Burmasov S.P., Gudov A.G., Smirnov L.A. On the possibility of increasing the operational properties of steel by technological influences on structural state of iron melts. In: Influence of the Properties of the Metal Matrix on the Operational Durability of Rails: Proceedings. Yekaterinburg: JSC UIM; 2006:9–17. (In Russ.).
About the Authors
A. G. GudovRussian Federation
Aleksandr G. Gudov, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Metallurgy of Iron and Alloys”
19 Mira Str., Yekaterinburg 620002, Russian Federation
S. P. Burmasov
Russian Federation
Sergei P. Burmasov, Cand. Sci. (Eng.), Assist. Prof.
19 Mira Str., Yekaterinburg 620002, Russian Federation
L. A. Smirnov
Russian Federation
Leonid A. Smirnov, Academician, Dr. Sci. (Eng.), Prof., Scientific Director
14 Gagarina Str., Yekaterinburg 620062, Russian Federation
Review
For citations:
Gudov A.G., Burmasov S.P., Smirnov L.A. Formation of iron-carbon melts and technological aspects of carburization. Izvestiya. Ferrous Metallurgy. 2025;68(5):506-516. https://doi.org/10.17073/0368-0797-2025-5-506-516