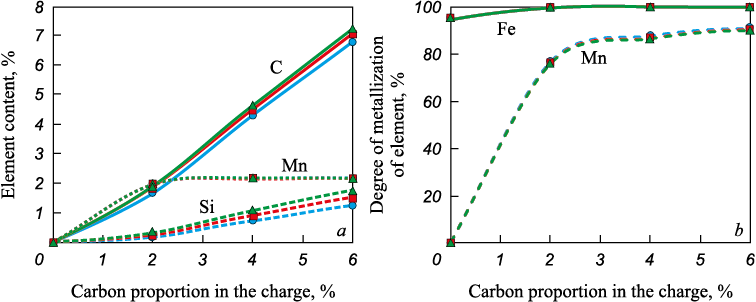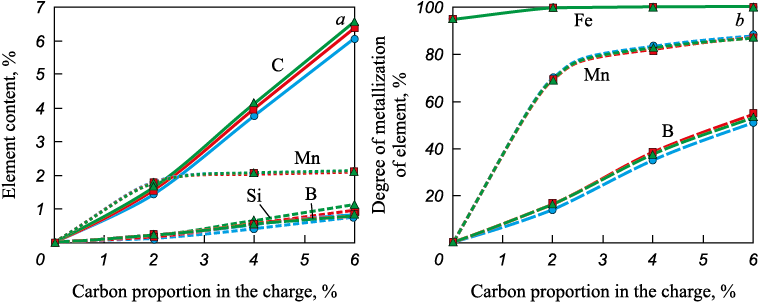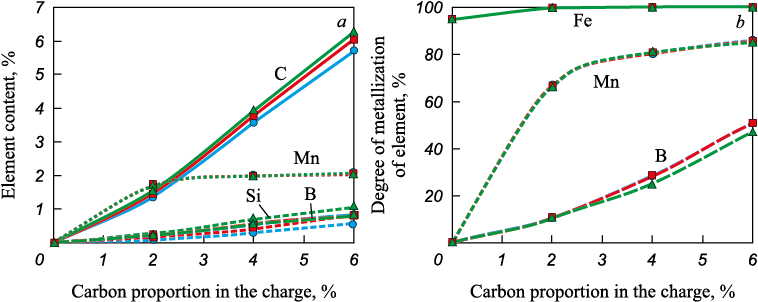Scroll to:
Boron distribution between metal and slag during melting of metalized siderite concentrate in electric furnace
https://doi.org/10.17073/0368-0797-2025-5-495-505
Abstract
The Ural metallurgical enterprises compensate for the deficit of iron ore raw materials by supplying materials from Central Russia, the Kola Peninsula and Kazakhstan. Carbonate iron ores (siderites) of the Bakalskoye deposit are poor ores and, despite large reserves (about 1 billion tons), are not in great demand among metallurgists due to their low quality (low iron content and high magnesium content). The prospects for developing the Bakalskoye deposit depend on the availability of new technologies for processing siderites. There is a technology for processing poor iron ores using the coke-free metallurgy method, including reducing roasting in a rotary kiln, grinding and magnetic separation to obtain a highly metallized product suitable for steelmaking. Laboratory studies and industrial tests confirmed its suitability for processing siderites. A modernized technology for processing siderites is proposed, in which the operations of grinding and magnetic separation are excluded, and the product of reducing roasting in a rotary kiln is loaded hot into an electric furnace for separating melting. The process is carried out in the presence of colemanite containing boric anhydride to obtain liquid slag. During melting, part of the boron passes into the metal melt. By means of thermodynamic modeling, an assessment of the distribution of boron between the metal and slag as a result of separating melting is carried out. With a content of 5 and 10 % of colemanite in the charge, depending on the proportion of carbon, up to 60 % of boron passes into the metal. Such metal can be used as a ligature for obtaining boron-containing steel or cast iron. When bubbling the metal melt with oxygen, boron content can be reduced to 0.0001 %.
For citations:
Vusikhis A.S., Tyushnyakov S.N., Pikulin K.V., Agafonov S.N., Leont’ev L.I. Boron distribution between metal and slag during melting of metalized siderite concentrate in electric furnace. Izvestiya. Ferrous Metallurgy. 2025;68(5):495-505. https://doi.org/10.17073/0368-0797-2025-5-495-505
Introduction
The reserves of carbonate iron (siderite) ores at the Bakalskoye deposit located near the town of Bakal in the Chelyabinsk Region are estimated at about 1 billion tons [1; 2]. However, the Ural metallurgical enterprises compensate for the deficit of iron ore raw materials by supplying materials from Central Russia (Mikhailovsky Mining and Processing Plant, Lebedinsky Mining and Processing Plant), the Kola Peninsula (Kostomuksha Mining and Processing Plant), and Kazakhstan (Sokolov–Sarbai Mining and Processing Plant) [3; 4]. Bakal siderites are classified as poor iron ores, containing less than 35 % iron and a large proportion of magnesium oxide. The low quality of the ore limits its demand; therefore, the production of siderite ore is much lower than the potential allowed by the mining and geological conditions. For example, in 2006, ore production amounted to 1.7 million tons, or less than 3 % of the total output in the Ural Federal District [5]. The only industrially implemented method for processing Bakal siderites is blast-furnace melting [1; 2]. Existing beneficiation methods [6; 7] make it possible to increase the iron content in the obtained concentrate. However, the entire amount of magnesium oxide remains in the concentrate, which restricts its use in blast-furnace smelting to the role of a charge additive and only in quantities ensuring that the magnesium oxide content in the final slag does not exceed 20 %. Therefore, the prospects for developing the Bakalskoye deposit depend on the availability of new technologies for processing siderites.
To expand the range of siderite applications and increase their processing efficiency, a pyrometallurgical beneficiation technology has been developed [2; 8; 9]. It includes reducing roasting of siderites in a rotary kiln using a solid reducing agent, followed by magnetic separation to obtain a highly metallized product. The resulting concentrate contains less than 5 % of gangue and, when briquetted, can serve as a feed material for an electric-arc furnace.
A process was proposed to reduce costs by eliminating grinding, magnetic separation, drying, and briquetting, in which hot metallized lump concentrate is fed directly from the rotary kiln to the electric furnace instead of using briquettes [10]. Comparative analysis of the two melting routes showed that the specific power consumption for melting metallized briquettes loaded into the furnace at 25 °C amounts to 773 kW·h per ton of metal, whereas melting hot metallized lump concentrate at 1000 °C requires 725 kW·h per ton of metal [11].
It is well known that the addition of boric anhydride to slag lowers its melting temperature [12; 13]. Since the gangue in the metallized concentrate contains a large amount of magnesium oxide, boron oxide in the form of calcined colemanite is added to the charge to produce a liquid, free-flowing slag with a low melting temperature. This provides a slag viscosity of less than 0.4 Pa·s at 1600 °C and a melting temperature of 1300 – 1500 °C, depending on the colemanite content in the charge and the SiO2/MgO ratio in the slag [14].
When boron oxide is present in the steelmaking slag, boron can pass into the metal [15]. this study uses thermodynamic modeling to evaluate how boron partitions between metal and slag during separation melting of metallized siderite concentrate in the presence of colemanite.
Materials and methods
Metallurgical processes occur at high temperatures and proceed at high rates; therefore, it can be assumed with sufficient confidence that during the melting of siderites the system reaches a state close to equilibrium. Consequently, to evaluate boron distribution between the metal and slag, the thermodynamic modeling (TDM) method was applied. This method is widely used to calculate multicomponent and multiphase systems when solving both theoretical and applied problems related to improving metallurgical technologies. Modeling of equilibrium states in the Fe – Ca – Si – Mg – Al – Mn – C – O system was performed using the IVTANTHERMO software package. Numerical values of the total enthalpy of the resulting equilibrium compositions were obtained using HSC Chemistry 6.12.
In siderite ores of the Bakal ore field, subjected to preliminary gravitational or X-ray radiometric beneficiation [16] for shale removal, the contents of most components differ only slightly. The main difference lies in the SiO2/MgO ratio [17; 18]. Therefore, a concentrate of the following composition (wt. %) was taken as the initial material for the study: 33.08 Fetot ; 38.94 FeO; 2.27 Fe2O3 ; 1.41 CaO; 0.66 SiO2 ; 11.22 MgO; 0.38 Al2O3 ; 0.85 Mn. The SiO2/MgO ratio was adjusted by adding quartz. The final compositions of the model concentrates are presented in Table 1.
Table 1. Compositions of the initial model concentrates used
| |||||||||||||||||||||||||||||||||||||||||||||||||
The masses (mc ) and compositions of the metallized siderite concentrates (MSC) with a metallization degree of φ = 95 %, obtained from 1 kg of initial concentrate, are given in Table 2.
Table 2. Masses and compositions of MSCs with a metallization degree of 95 %
| ||||||||||||||||||||||||||||||||||||||||||||||||
As a fluxing additive used to liquefy the slag, calcined colemanite [19] with the following composition (wt. %) was employed: 8 SiO2 ; 34 CaO; 4 MgO; and 54 B2O3 . In practice, the solid reducing agent (coke breeze) with a particle size of 3 – 5 mm is separated from MSC lumps sized 10 – 60 mm by screening. However, some portion of the reducing agent may enter the electric furnace together with the concentrate; therefore, it was assumed in the calculations that the electric-furnace charge contains carbon.
Thus, the model systems used for calculating phase equilibria consisted of MSC (Table 2), colemanite, and carbon, taken in amounts of 0, 5, and 10 wt. % and 0, 2, 4, and 6 wt. %, respectively, relative to the mass of metallized concentrate. The modeling was carried out at a temperature of 1600 °C, pressure of 0.1 MPa, and under the assumption that the oxide and metallic phases behave as ideal solutions.
Results and discussion
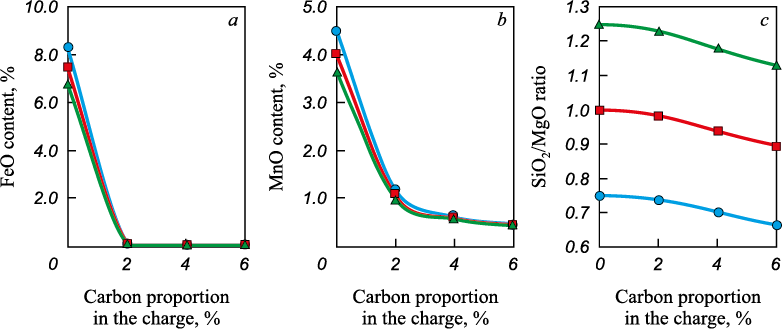
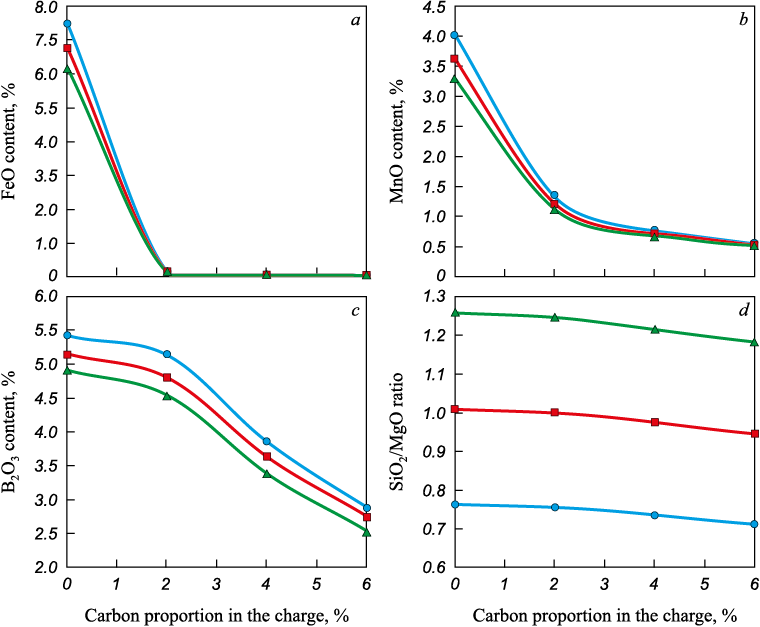
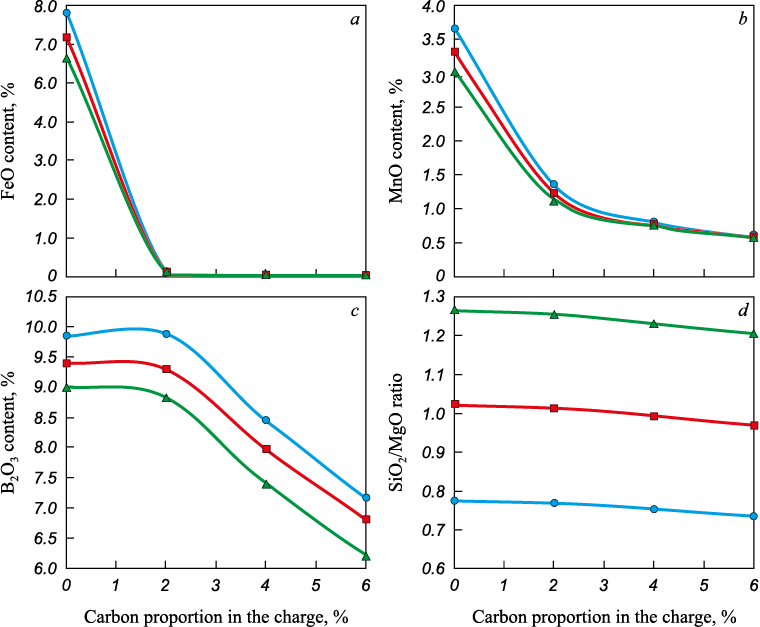
Equilibrium calculations for the MSC–carbon system show that in the presence of carbon, the amounts of iron, manganese, and silicon oxides in the oxide phase decrease (Fig. 1), and these elements pass into the metal together with residual carbon (Fig. 2).
Fig. 1. Dependence of content of FeO (a), MnO (b), SiO2/MgO ratio (c)
Fig. 2. Dependence of content of carbon, manganese and silicon in the metal (a) |
At a temperature of 1600 °C, corresponding to the melting temperature, the iron-based metal exists in a liquid state. To estimate the crystallization onset temperature of the slags obtained from equilibrium calculations, two methods were applied.
The first method was based on the results reported in [14], which analyzed the effect of the SiO2/MgO ratio and the amount of colemanite in the charge on the transition temperature of slags from their high-temperature stability region to the low-temperature region, close to the liquidus temperature. The dependence is described by the following empirical equation:
T = 1801.7 – 199.5x – 28.6x2 – 7.19y + 0.03y2 – 4.0xy,
where х is the SiO2/MgO ratio (units); y is the colemanite content (wt. %).
It was established that in the absence of colemanite in the charge, the transition temperature decreases from 1640 to 1510 °C as the SiO2/MgO ratio increases from 0.75 to 1.25.
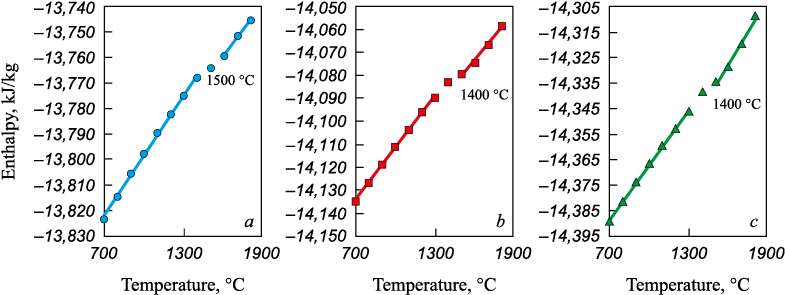
A similar result was obtained using the second method described in [20], according to which the temperatures of the beginning (ts ) and end (tl ) of melting (solidus and liquidus, respectively) were determined from inflection points on the total entropy and enthalpy curves characterizing the system state. This approach was used as the basis for liquidus temperature calculations of the slag systems considered in this study.
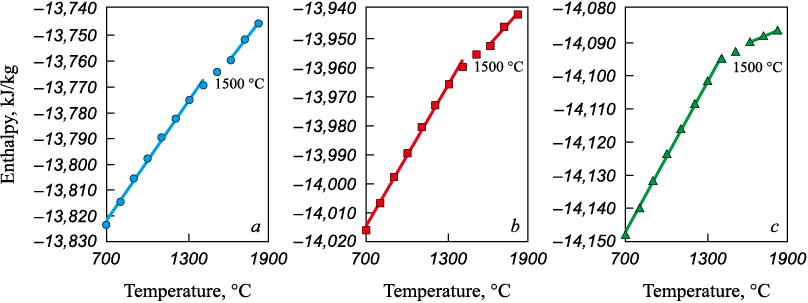
The slags used as input were those whose compositions were determined from the equilibrium calculations in the MSC–carbon system. The results showed that in slags without carbon and with SiO2/MgO = 0.75 – 1.25, tl lies in the range of 1450 – 1600 °C (Fig. 3). In slags formed as a result of the interaction between MSC and carbon, a decrease in FeO content – caused by an increase in the carbon proportion – does not lead to a further increase in tl (Fig. 4).
Fig. 3. Dependence of the system total enthalpy on temperature for slag systems
Fig. 4. Dependence of the system total enthalpy on temperature for slag systems calculated |
Thus, the calculated crystallization onset temperatures of oxide phases formed during the interaction of MSC with carbon indicated that at 1600 °C, corresponding to the electric furnace temperature, these phases remain heterogeneous. This confirms the need for a flux additive based on boric anhydride to liquefy the slag, for which colemanite was chosen.
Equilibrium calculations for the MSC–colemanite–carbon system showed the following. The addition of carbon increases the manganese and silicon content in the metal and decreases the amount of iron, manganese, and silicon oxides in the slag phase, similar to the MSC–carbon system (Figs. 5 – 8). In addition, boron passes into the metal, accompanied by a decrease in boron oxide content in the slag.
Fig. 5. Dependence of content of carbon, manganese, boron and silicon in the metal (а)
Fig. 6. Dependence of content in the oxide phase of FeO (a), MnO (b), B2O3 (c),
Fig. 7. Dependence of content of carbon, manganese, boron and silicon in the metal (а)
Fig. 8. Dependence of content in the oxide phase of FeO (a), MnO (b), B2O3 (c), |
In the absence of carbon, the oxide phase compositions are close to those of the slags investigated in [14], where, as the SiO2/MgO ratio increased from 0.75 to 1.25, tl decreased from 1520 to 1370 °C, and the viscosity of all slags was below 0.3 Pa·s.
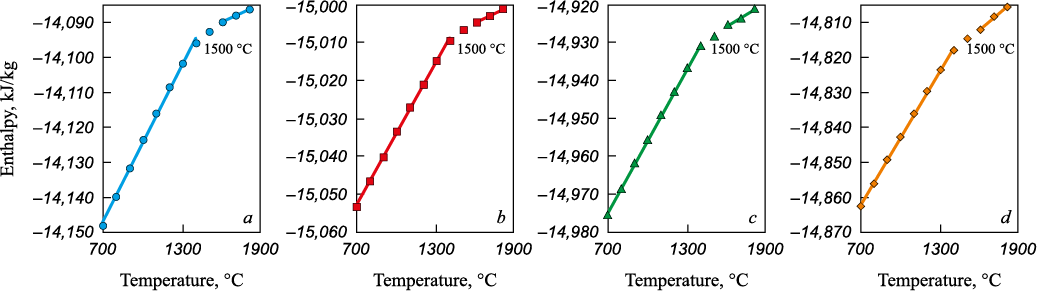
Evaluation of phase transition temperatures in the MSC–colemanite–carbon systems (Fig. 9) indicates a decrease in the liquidus temperature from 1500 to 1400 °C when 5 – 10 wt. % colemanite is added to the charge. This suggests that separation melting of metallized siderite concentrate in the presence of colemanite is feasible even when carbon is present in the charge. According to theoretical calculations, the slag in this case will have a sufficiently low melting temperature, allowing it to be removed from the furnace after separation melting from the metal at 1600 °C. Depending on the carbon content in the charge, the metal will contain manganese, silicon, and boron.
Fig. 9. Dependence of the system total enthalpy on temperature for slag systems |
Small additions of boron are known to improve the quality of cast iron [21; 22], steel [23 – 25], and surfacing materials [26]. They significantly refine the grain structure. Strengthening of grain boundaries by borides enhances high-temperature strength, as well as increased hardness and wear resistance, and sharply improves hardenability compared to similar steels without boron. Microadditions of boron (about 0.01 % in cast irons and 0.001 – 0.003 % in steels) can replace alloying elements such as nickel, chromium, molybdenum, and manganese in amounts 300 – 400 times greater than that of boron, while maintaining metal quality.
In the proposed technology, the amount of boron transferred to the metal is significantly higher than that required for producing quality cast iron or steel. Therefore, the resulting metal can be used as a master alloy, or its boron content should be substantially reduced, for example, by bubbling oxidation with oxygen.
To assess the feasibility of bubbling oxidation, additional thermodynamic modeling was performed using the methodology previously applied for modeling bubbling reduction of metals from oxide melts [27 – 29]. The initial model system consisted of metal with the following composition (%): 96.1 Fe; 1.7 C; 0.20 Si; 1.80 Mn; and 0.26 B (1 kg). The gas phase was oxygen, supplied in an amount of 7.3 dm3 per kilogram of metal. Modeling was carried out at 1600 °C and 1 atm, assuming the melt to be an ideal solution.
The calculations were performed in the following sequence:
– input of initial data on the amount of metal melt and oxygen;
– equilibrium calculation of the system using thermodynamic modeling methods;
– recording equilibrium compositions and the amounts of components in the oxide and metallic melts, as well as in the gas phase;
– performing the next cycle, in which the composition of the metallic melt obtained in the previous step was taken as the initial state, while the slag was considered removed from the system and not accounted for; the gas composition and amount remained unchanged;
– repeating the cycles until the amount of oxidized components in the melt decreased to the specified level.
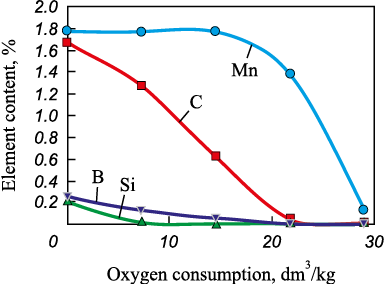
Fig. 10 shows the change in metal composition depending on oxygen consumption.
Fig. 10. Dependence of the components content in the metal |
As a result of bubbling oxidation of the metal, the iron content increases to 99.86 %, the manganese content decreases to 0.13 %, silicon is completely oxidized, and boron content drops to 0.000002 %. From 1 kg of the initial metal, 940 g of final metal and 201 g of slag are formed, with the slag composition (wt. %) as follows: 46.7 FeO; 6.7 SiO2 ; 34.3 MnO; 12.4 B2O3 . This slag can be used together with colemanite as an additional source of boron.
Conclusions
Thermodynamic modeling of the separation melting of metallized siderite concentrate with colemanite in the presence of carbon made it possible to describe the distribution of boron between the metal and slag depending on the proportions of colemanite and carbon in the system. It is shown that adding 5 – 10 % colemanite to the charge reduces the slag melting temperature to 1400 °C, enabling efficient extraction of both metal and slag from the furnace at 1600 °C. With an increase in carbon content in the charge up to 6 %, a linear dependence of the degree of boron transfer to the metal was observed, reaching 60 %. The resulting boron-containing metal can be used as a master alloy for microalloying cast irons and steels, or as a semi-finished metal product suitable for secondary (ladle) treatment after preliminary oxygen bubbling. The slag produced during bubbling can be reused as an additional boron-bearing component of the charge. The results of the calculations can serve as a basis for planning and conducting experimental studies on the processes of separation melting of metallized siderite concentrates.
References
1. Krasnoborov V.A., Yaroshevskii S.L., Denisov A.A., Rudin V.S., Biryuchev V.I., Polushkin M.F. Efficiency and Prospects of Using Siderite Ores in Blast Furnace Melting. Donetsk: Novyi Mir; 1996:74. (In Russ.).
2. Yur’ev B.P., Melamud S.G., Spirin N.A., Shatsillo V.V. Technological and Thermal Engineering Principles of Preparation of Siderite Ores for Metallurgical Processing. Yekaterinburg: Den’ RA; 2016:428. (In Russ.).
3. Volkov Yu.V., Sokolov I.V., Smirnov A.A. Strategy for development of raw material resources of the Urals. Gornaya promyshlennost’. 2006;(4):57–62. (In Russ.).
4. Volkov Yu.V., Slavikovskii O.V., Sokolov I.V., Smirnov A.A. Prospects for development of raw material base of mining and metallurgical enterprises of the Urals. GIAB. 2007; (5):286–290. (In Russ.).
5. Pakhomov V.P., Dushin A.V. Assessment of mineral resource security of the Urals Federal District. Ehkologiya regiona. 2008;(3):129–143. (In Russ.).
6. Zhunev A.G., Fedorenko N.V., Chervotkin V.V. Oxidative roasting of siderite ores in shaft furnaces. In: Pelletizing of Iron Ores and Concentrates: Proceedings of the Institute “Uralmekhanobr”. Sverdlovsk; 1976(3):28–38. (In Russ.).
7. Blank M.E., Chervotkin V.V., Konchakovskii V.R. Development and study of a two-stage method of iron ore raw materials metallization in a three-zone shaft furnace. In: Theory and Practice of Direct Iron Production. Moscow: Nauka; 1986:207–211. (In Russ.).
8. Melamud S.G., Shatsillo V.V., Dudchuk I.A., Mushketov A.A., Bratygin E.V., Yur’ev B.P. Enrichment of reduced siderite ore to produce concentrate for electrosteel smelting. Steel in Translation. 2011;41(6):492–498. https://doi.org/10.3103/S0967091211060076
9. Vusikhis A.S., Sheshukov O.Yu., Leont’ev L.I. Method of metallization of magnesium-containing carbonate iron ore materials. Patent RF no. 2489494. Byulleten’ izobretenii. 2013;(22). (In Russ.).
10. Vusikhis A.S., Leont’ev L.I. Method for processing magnesium-containing carbonate iron ore materials. Patent RF no. 2820696. Byulleten’ izobretenii. 2024;(16). (In Russ.).
11. Vusikhis A.S., Leon’yev L.I., Chesnokov Yu.A. Evaluating the efficiency of metallized siderite concentrate electric melting. Izvestiya. Ferrous Metallurgy. 2023;66(6):653–658. https://doi.org/10.17073/0368-0797-2023-6-653-658
12. Hongming W., Tingwing Z., Hua Z., Li G., Yan Y., Wang J. Effect of B2O3 on melting temperature, visocity and desulfurization capacity of CaО-based refining flus. ISIJ International. 2011;51(5):702–708. https://doi.org/10.2355/isijinternational.51.702
13. Ren Sh., Zhang J., Wu L., Liu W., Bai Y., Xing X., Su B., Kong D. Influence of B2O3 on viscosity of high Ti-bearing blast furnace slag. ISIJ International. 2012;52(6):984–991. https://doi.org/10.2355/isijinternational.52.984
14. Vusikhis A.S., Leont’ev L.I., Mikheenkov M.A. Effect of boric anhydride on viscosity of slags used in electric melting of metallized siderite concentrate. Izvestiya. Ferrous Metallurgy. 2023;66(5):597–603. https://doi.org/10.17073/0368-0797-2023-5-597-603
15. Zhuchkov V.I., Salina V.A., Sychev A.V. The study of the process of metal-thermal reduction of boron from the slag of the system СаО–SiO2–MgO–Al2O3–B2O3 . Materials Science Forum. 2019;946:423–429. http://doi.org/10.4028/www.scientific.net/MSF.946.423
16. Zagainov S.A., Yur’ev B.P. Dressing Processes and Devices for Their Implementation. Yekaterinburg: UrFU; 2012:110. (In Russ.).
17. Timeskov V.A. Mineralogy of Carbonate Ores and Host Carbonate Rocks of the Bakal Iron Ore Deposit in the Southern Urals. Kazan: Kazan University; 1963:214. (In Russ.).
18. Vusikhis A.S., Leont’ev L.I. Use of Siderite Ores in Production of Iron and Steel. Moscow; Vologda: Infra-Engineering; 2022:116. (In Russ.).
19. Öztürk Ç., Akpınar S., Tığ M. Effect of calcined colemanite addition on properties of porcelain tile. Journal of the Australian Ceramic Society. 2022;58:321–331. https://doi.org/10.1007/s41779-021-00674-2
20. Marshuk L.A., Vusikhis A.S., Leont’ev L.I. Study of physicochemical characteristics of oxide systems CaO–SiO2–Al2O3–MgO by thermodynamic modeling. In: Proceedings of the VI Congress with Int. Participation “Technogen-2023”, July 11–14, 2023. Yekaterinburg; 2023:361–363. (In Russ.).
21. Kolokol’tsev V.M., Petrochenko E.V., Molochkova O.S. Influence of boron modification and cooling conditions during solidification on structural and phase state of heat- and wear-resistant white cast iron. Izvestiya. Ferrous Metallurgy. 2019;62(11):887–893. (In Russ.). https://doi.org/10.17073/0368-0797-2019-11-887-893
22. Gimaletdinov R.Kh., Gulakov A.A., Tukhvatulin I.Kh. Influence of chemical composition on the properties of the working layer of centrifugally cast indefinite rolling rolls. Bulletin of Magnitogorsk State Technical University named after G.I. Nosov. 2016;14(3):78–89. (In Russ.).
23. Kel’ I.N., Zhuchkov V.I., Sychev A.V. Application of boron-containing materials in ferrous metallurgy. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2018;(5):48–53. (In Russ.).
24. Nazyuta L.Yu., Tsurkan M.L., Tikhonyuk L.S., Tarasenko O.S., Khavalits Yu.V. Influence of boron on technological properties of medium-carbon structural steels of ordinary assortment. Visn. priazov. state. technical. un-tu. 2018;(37):22–28. (In Russ.).
25. Baradyntseva E.P., Glazunova N.A., Rogovtsova O.V. Effect of boron microalloying on hardenability of steels. Lit’e i metallurgiya. 2016;(3(84)):70–74. (In Russ.).
26. Lyamin A.V., Madrakhimov D.U. Influence of boron on phase state and properties of surfacing materials. Molodoi uchenyi. 2021;(4(346)):24–26. (In Russ.).
27. Vusikhis A.S., Leont’ev L.I., Selivanov E.N., Chentsov V.P. Modeling the process of gas reduction of metals from a multicomponent oxide melt in bubbling layer. Butlerovskie soobshcheniya. 2018;55(7):58–63. (In Russ.).
28. Vusikhis A.S., Dmitriev A.N., Kudinov D.Z., Leontiev L.I. The study of liquid and gas phases interaction during the reduction of metal oxides from the melts by gas reductant in bubbled layer. In: The Third Int. Conf. on Mathematical Modeling and Computer Simulation of Materials Technologies (MMT-2004), Ariel, Israel. 2004;1:72–77.
29. Dmitriev A., Leontiev L., Vusikhis A., Kudinov D. Liquid and gas interaction during reduction in bubbled layer. In: Proceeding of the European Metallurgical Conference (EMC-2005). Dresden, Germany. 2005;3:1349–1358.
About the Authors
A. S. VusikhisRussian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
S. N. Tyushnyakov
Russian Federation
Stanislav N. Tyushnyakov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
K. V. Pikulin
Russian Federation
Kirill V. Pikulin, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
S. N. Agafonov
Russian Federation
Sergei N. Agafonov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. I. Leont’ev
Russian Federation
Leopol’d I. Leont’ev, Academician, Adviser, Presidium of the Russian Academy of Sciences; Dr. Sci. (Eng.), Prof., National University of Science and Technology “MISIS”; Chief Researcher, Vatolin Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
32a Leninskii Ave., Moscow 119991, Russian Federation
Review
For citations:
Vusikhis A.S., Tyushnyakov S.N., Pikulin K.V., Agafonov S.N., Leont’ev L.I. Boron distribution between metal and slag during melting of metalized siderite concentrate in electric furnace. Izvestiya. Ferrous Metallurgy. 2025;68(5):495-505. https://doi.org/10.17073/0368-0797-2025-5-495-505