Scroll to:
Features of application of boron-containing slags in stainless steel smelting
https://doi.org/10.17073/0368-0797-2025-5-488-494
Abstract
Traditionally, for stainless steel smelting in the process of argon-oxygen decarburization, fluorspar is used to liquefy the slag and ensure the normal course of the refining and reduction of chromium oxide. Fluospar is characterized by high volatility at high temperatures of the steelmaking process, while the resulting compounds are toxic and hazardous to the environment. For this reason, the paper considers the replacement of fluorspar with boron oxide, which is also capable of forming low-melting eutectics with the main components of slag, at the final stage of steel processing during the argon-oxygen decarburization process – during the desulfurization period. It was found that, despite an increase in the degree of slag polymerization as a result of the introduction of boron oxide to 6 %, due to its ability to form low-melting compounds, an increase in its content has a beneficial effect on the fluidity of slags of the studied СаО – SiO2 – В2O3 – 2 % Cr2O3 – 3 % Аl2O3 – 8 % МgO system at a basicity of CaO/SiO2 of 1.0 and 2.5. The content of 6 % B2O3 in slag with a high basicity of 2.5 makes it possible to achieve viscosity values of 0.3 Pa·s, which are favorable for sulfur removal. In this case, the equilibrium sulfur content in the metal can reach 0.003 % according to the thermodynamic modeling. As a result of the experimental studies, the minimum sulfur content was 0.006 %, which is close to the equilibrium concentration. During the treatment of steel samples with slags, direct steel microalloying with boron in the amount of 0.002 – 0.003 % occurred. A small amount of boron transferred to steel during direct microalloying, according to literary data, has a beneficial effect on the ductility and corrosion resistance of the metal product.
Keywords
For citations:
Babenko A.A., Shartdinov R.R., Lobanov D.A., Smetannikov A.N., Upolovnikova A.G., Gulyakov V.S. Features of application of boron-containing slags in stainless steel smelting. Izvestiya. Ferrous Metallurgy. 2025;68(5):488-494. https://doi.org/10.17073/0368-0797-2025-5-488-494
Introduction
At present, the argon–oxygen decarburization (AOD) process [1; 2] is widely used in the smelting of low-carbon stainless steels. The process proceeds in two stages: the oxidation and reduction periods. During the first stage, carbon is oxidized; during the second, chromium, which has been oxidized in the first period, is reduced. When deep desulfurization is required at the end of the reduction period, most of the existing slag is skimmed, and a new highly basic slag with a low chromium oxide content is introduced [1]. The desulfurization reaction is limited by mass transfer in the slag; therefore, the slag is traditionally liquefied with environmentally hazardous fluorspar [1; 3; 4]. As a replacement for fluorspar, boron oxide can be used, as it is also capable of forming low-melting eutectics with calcium oxide [5 – 7].
In this work, four slags of the СаО – SiO2 – В2O3 – 2 % Cr2O3 – 3 % Аl2O3 – 8 % МgO system, close in composition to slags of the desulfurization period, were prepared. Experimental studies of the viscosity, crystallization onset temperature, and structure of these slags were performed, as well as thermodynamic modeling and experimental investigation of the desulfurization of metal under slags of this oxide system.
Materials and methods
The physicochemical characteristics of four slags were investigated in this work. Their compositions are presented in Table 1. The basicity of the slags (Bslag = CaO/SiO2 ) was 1.0 and 2.5, and the boron oxide content was 0 and 6 wt. % B2O3 .
Table 1. Composition of experimental slags and results of modeling
| |||||||||||||||||||||||||||||||||||||||||||||||||
Synthetic slags were melted in molybdenum crucibles from analytically pure grade oxides that had been pre-calcined for 2 – 3 h at 800 °C (B2O3 at 105 °C) and thoroughly mixed. The obtained homogenized slag samples were crushed to produce powder.
The viscosity of the slags was measured by the electro-vibrational viscometry method [8] in a resistance furnace during gradual cooling of the melt contained in molybdenum crucibles under an argon atmosphere. Temperature was monitored with a tungsten–rhenium thermocouple W–5%Re/W–20%Re (WR5/20). The crystallization onset temperature (hereinafter, the crystallization temperature, was determined from the break (kink) in the viscosity polytherm plotted as ln η vs 1/T [9].
Thermodynamic modeling of the phase composition and equilibrium sulfur content was performed using the HSC Chemistry 6.12 software package [10]. The chemical compositions of the four slag samples are presented in Table 1. The metal contained (wt. %): 16.5 Cr, 0.02 C, 0.6 Si, 0.03 S, 1.6 Mn, 8.4 Ni, and 0.006 Al. The results of the calculated equilibrium sulfur concentration in the metal ([S]calc ) at 1600 °C are summarized in Table 1. The phases were conventionally grouped by melting point into low-, medium-, and high-temperature categories, as presented in Table 2.
Table 2. Phase composition of the studied slags at 1600 °C
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Experimentally, the desulfurization process and the reduction behavior of boron were studied by holding steel under pre-melted slags 1 – 4 (Table 1) in magnesia crucibles for 10 – 60 min at 1600 °C in an Ar atmosphere. Each experimental charge (sample) consisted of 80 g of metal and 16 g of slag.
The structure of the experimental slag samples was examined by Raman spectroscopy using a U 1000 Raman micro-spectrometer with an excitation laser wavelength of 532 nm.
Results and discussion
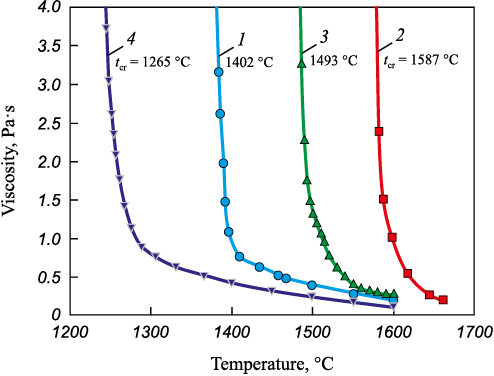
The results of viscosity measurements for the four studied slags, presented in Fig. 1, confirmed the high efficiency of boron oxide as a flux. The addition of 6 wt. % B2O3 markedly reduced both the viscosity and the crystallization onset temperature (tcr ) at low and high basicity.
Fig. 1. Viscosity-temperature dependence of slags 1 – 4 |
To clarify the mechanism of boron oxide action and the processes occurring in the slag upon its addition, the phase composition and structural features of the slags were examined using thermodynamic modeling and Raman spectroscopy.
The results showed that the presence of boron oxide promotes the formation of a considerable amount of low-melting compounds, primarily various calcium borates, accompanied by a pronounced decrease in the content of free CaO (Table 2).
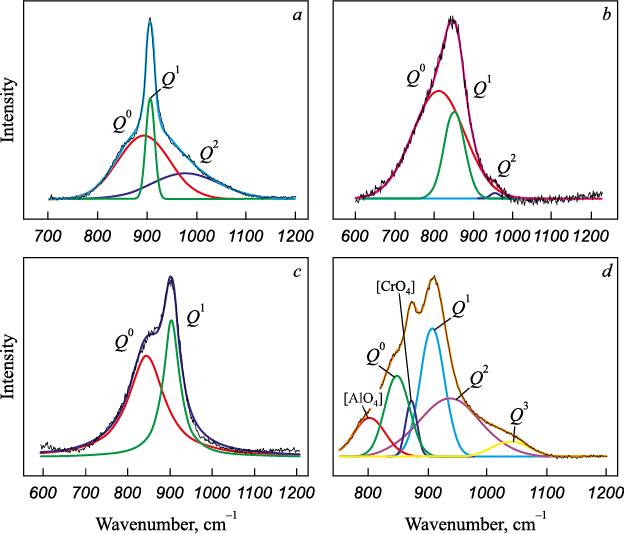
To assess the influence of boron oxide on slag structure, Raman spectra were recorded for the slags, and their deconvolution was performed using the Gaussian method [11] in the silicate range of 800 – 1200 cm–1. This approach made it possible to express the degree of slag polymerization through the average number of bridging oxygen (BO) (Fig. 2, Table 3):
\[{\rm{BO}} = 0 \cdot Q_{{\rm{Si}}}^0 + 1 \cdot Q_{{\rm{Si}}}^1 + 2 \cdot Q_{{\rm{Si}}}^2 + 3 \cdot Q_{{\rm{Si}}}^3 + 4 \cdot Q_{{\rm{Si}}}^4,\]
where \(Q_{{\rm{Si}}}^n\) denotes [SiO4 ] tetrahedra with n bridging oxygen (O0).
Fig. 2. Deconvoluted spectra of slag samples 1 – 4
Table 3. Decovolution results and polymerization degree BO
| |||||||||||||||||||||||||||||||||||||
In Fig. 2, peaks corresponding to [SiO4 ] tetrahedra with up to three (O0) appear in the wavenumber range 850 – 1060 cm–1 [12; 13], peaks of [CrO4 ] groups occur at 873 cm–1 [14], and those of \(Q_{{\rm{Al}}}^3\) ([AlO4 ]) at 780 cm–1 [15]. Three-dimensional [BO4 ] tetrahedra are located in the range of 900 – 920 cm–1 [16; 17] and overlap with the [SiO4 ] peaks.
When boron oxide is introduced into low-basicity slags (B = 1.0), the degree of polymerization increases from 0.73 to 1.28 due to an increase in the fractions \(Q_{{\rm{Si}}}^1\), \(Q_{{\rm{Si}}}^2\) and species and the formation of \(Q_{{\rm{Si}}}^3\) species at the expense of \(Q_{{\rm{Si}}}^0\) species (Table 3). Boron oxide acts as a network former and complicates the slag structure. Additional polymerization is also indicated by the appearance of [CrO4 ] and \(Q_{{\rm{Al}}}^3\) peaks in spectrum 4, which are absent in spectrum 1.
An increase in basicity to 2.5 – and, consequently, in the CaO content of the slag, which acts as a donor of free oxygen ions (O2–), inevitably breaks down the previously formed silicate structures, so the degree of polymerization (BO) decreases markedly to 0.26 (slag 2) and 0.38 (slag 3) for 0 and 6 wt. % B2O3 , respectively. Under high basicity and an increased concentration of (O2–) boron oxide polymerizes the slag to a much lesser extent, primarily through an increase in the fraction of \(Q_{{\rm{Si}}}^1\) species.
The high basicity of slags 2 and 3 (Bslag = 2.5) also leads to higher viscosity, despite their simpler structure compared to slags of lower basicity (BO decreases from 0.73 to 0.26 and from 1.28 to 0.38 for slags without and with 6 wt. % B2O3 , respectively). The viscosity of slag 3 (with 6 wt. % B2O3 ), although having a relatively simple structure (BO = 0.38), sharply increases to 1.3 Pa·s at 1500 °C, together with its crystallization onset temperature of 1493 °C. This can be explained by the much smaller fraction of low-melting phases (5.4 %) and the increased proportion of high-melting ones (up to 50.8 %), represented mainly by 2CaO·SiO2 with a melting point of 2130 °C. The highly basic slag 2, which does not contain boron oxide, is the most viscous and refractory in the system. Its crystallization onset temperature is 1587 °C, and its viscosity, despite a relatively simple structure (BO = 0.26), reaches 0.75 Pa·s at 1600 °C. This behavior is associated with the highest content of high-melting compounds (78.4 %), mainly 2CaO·SiO2 and free CaO.
Despite the high degree of polymerization (BO = 1.28), slag 4 (Bslag = 1.0, 6 wt. % B2O3 ) shows low viscosity due to its highest content of low-melting phases (23 %). The viscosity ranges from 0.40 to 0.25 Pa·s within 1400 – 1500 °C, and its crystallization onset temperature is 1265 °С.
Slag 1 with the same basicity but without boron oxide has a much simpler structure (BO = 0.73), but its viscosity is higher – 0.9 – 0.4 Pa·s in the 1400 – 1500 °C range – and its crystallization onset temperature rises to 1402 °C. This can be attributed to the reduction in the fraction of low-melting phases to 8.4 %. In the absence of calcium borates, the low-melting phases are represented exclusively by CaO·MgO·2SiO2 with a melting point of 1391 °С.
From the above results, it can be concluded that the effect of the balance between the phase composition and structure of the slags on their viscosity is clearly pronounced.
It is well established that higher basicity enhances desulfurization. However, the effectiveness of desulfurization is governed not only by the chemical activity of the oxide system but also by diffusion within the slag, which is limited by viscosity. Therefore, previous experiments involving holding the metal beneath a slag cover were carried out to confirm the influence of the kinetic factor [18]. Experiments involving the treatment of metal with boron-containing slags of basicity 1.0 and 2.5, with a maximum boron oxide content of 6 wt. %, showed rather high desulfurization efficiency (Table 4). According to thermodynamic modeling performed using the HSC Chemistry software package, the equilibrium sulfur content, [S]calc , can reach 0.002 – 0.003 %. The experimentally obtained sulfur contents, [S]exp , after holding the metal under the studied slags were close to equilibrium and amounted to 0.006 and 0.017 % for slags 3 and 4, respectively. The role of viscosity becomes evident when comparing the highly basic slags. In slag 3, the relatively low viscosity enabled the experimental sulfur level to approach the equilibrium value after 60 min of holding. This effect was not observed with the much more viscous slag 2, confirming diffusion-controlled nature of the process under those conditions.
Table 4. Sulfur and boron content in metal
|
A moderate reduction of boron by silicon (present in the metal at 0.3 wt. %) was also observed. The boron content in the metal, [B]exp , after the holding period was 0.002 – 0.003 % (Table 4). According to the literature, such a boron level in austenitic stainless steel improves both corrosion resistance and ductility [19; 20].
Conclusions
It was established that the addition of up to 6 wt. % B2O3 to the slag, although increasing the degree of structural polymerization (BO) from 0.73 to 1.28 at low basicity and from 0.26 to 0.38 at high basicity, ensures sufficiently high melt fluidity within the studied basicity range. This effect results from the tendency of boron oxide to form low-melting eutectics with the main slag components. The resulting highly basic slags, with a viscosity of about 0.3 Pa·s, a basicity of 2.5, and a B2O3 content of 6 wt. %, enable deep desulfurization of the metal, providing an equilibrium sulfur content of approximately 0.003 % according to thermodynamic modeling. Under desulfurization temperatures, these slags remain in the homogeneous liquid region and exhibit a crystallization onset temperature well below 1600 °C. Holding the metal beneath a slag cover reduced the sulfur content to about 0.006 %. During this treatment, 0.002 – 0.003 % boron was taken up by the steel. Published data indicate that this direct-microalloying level enhances both ductility and corrosion resistance.
References
1. Tokovoi O.K. Argon-Oxygen Decarburization of Stainless Steel: Monograph. Chelyabinsk: SUSU; 2015:250. (In Russ.).
2. Jalkanen H., Holappa L. Converter steelmaking. In: Treatise on Process Metallurgy. Vol. 3. Oxford: Elsevier; 2013:223–270. https://doi.org/10.1016/B978-0-08-096988-6.00014-6
3. Dyudkin D.A., Kisilenko V.V. Steel Production, in 3 vols. Vol. 3. Secondary Metallurgy. Moscow: Teplotekhnik; 2010:544. (In Russ.).
4. Visuri V.V., Mattila R., Kupari P., Fabritius T. A comparative study on refractory wear associated with fluxes for AOD slags. In: Proceedings of the 7th Int. Congress on Science and Technology of Steelmaking, June 13–15, 2018, Venice, Italy. 2018:13–15.
5. Wang H.-m., Li G.-r., Li B., Zhang X.-j., Yan Y.-q. Effect of B2О3 on melting temperature of CaO-based ladle refining slag. ISIJ International. 2010;17(10):18–22. https://doi.org/10.1016/S1006-706X(10)60177-X
6. Babenko A.A., Smirnov L.A., Upolovnikova A.G., Smetannikov A.N., Sychev A.V. Theoretical bases and technology of steel exhaustive metal desulfurization and direct microalloying with boron beneath basic boron-containing slags. Metallurgist. 2020;63(11-12):1259–1265. https://doi.org/10.1007/s11015-020-00937-6
7. Li Q., Yang Sh., Zhang Y., An Zh., Guo Zh.Ch. Effects of MgO, Na2O, and B2O3 on the viscosity and structure of Cr2O3-bearing CaO–SiO2–Al2O3 slags. ISIJ International. 2017;57(4):689–696. https://doi.org/10.2355/ISIJINTERNATIONAL.ISIJINT-2016-569
8. Shtengel’meier S.V., Prusov V.A., Bogechov V.A. Improvement of the technique of measuring viscosity using a vibration viscometer. Zavodskaya laboratoriya. 1985;(9):56–57. (In Russ.).
9. Voskoboinikov V.G., Dunaev N.E., Mikhalevich A.G., Kukhtin T.I., Shtengel’meier S.V. Properties of Blast Furnace Slags: Reference Book. Moscow: Metallurgiya; 1975:180. (In Russ.).
10. Roine A. Outokumpu HSC Chemistry for Windows. Chemical Reactions and Equilibrium Software with Extensive Thermochemical Database. Pori: Outokumpu research OY; 2002:269.
11. Mysen B.O., Virgo D., Scarfe C.M. Relations between the anionic structure and viscosity of silicate melts – a Raman spectroscopic study. American Mineralogist. 1980;65(7-8): 690–710.
12. McMillan P. Structural studies of silicate glasses and melts – applications and limitations of Raman spectroscopy. American Mineralogist. 1984;69(7-8):622–644.
13. Matson D.W., Sharma S.K., Philpotts J.A. The structure of high-silica alkali-silicate glasses. A Raman spectroscopic investigation. Journal of Non-Crystalline Solids. 1983; 58(2-3):323–352. https://doi.org/10.1016/0022-3093(83)90032-7
14. Weckhuysen B.M., Wachs I.F. Raman spectroscopy of supported chromium oxide catalysts. Determination of chromium–oxygen bond distances and bond orders. Journal of the Chemical Society, Faraday Transactions. 1996;92(11): 1969–1973. https://doi.org/10.1039/FT9969201969
15. Kim T.S., Park J.H. Structure-viscosity relationship of low-silica calcium aluminosilicate melts. ISIJ International. 2014;54(9):2031–2038. https://doi.org/10.2355/isijinternational.54.2031
16. Cochain B., Neuville D.R., Henderson G.S., McCammon C.A., Pinet O., Richet P. Effects of the iron content and redox state on the structure of sodium borosilicate glasses: A Raman, Mössbauer and Boron K‐Edge XANES spectroscopy study. Journal of the American Ceramic Society. 2012;95(3):
17. –971. https://doi.org/10.1111/j.1551-2916.2011.05020.x
18. Kim Y., Morita K. Relationship between molten oxide structure and thermal conductivity in the CaO–SiO2–B2O3 system. ISIJ International. 2014;54(9):2077–2083. https://doi.org/10.2355/isijinternational.54.2077
19. Babenko A.A., Shartdinov R.R., Lobanov D.A., Smetannikov A.N., Upolovnikova A.G. Physicochemical properties of the СаО–SiO2–B2O3–2%Cr2O3–3%Аl2O3–8%МgO slag system. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2023;79(9):736–744. (In Russ.). https://doi.org/10.32339/0135-5910-2023-9-736-744
20. Cetin M. Effect of boron added corrosion behavior of cast 304 stainless steel. Protection of Metals and Physical Chemistry of Surfaces. 2019;55:1217–1225. https://doi.org/10.1134/S2070205119060054
21. Moshkevich E.I., Travinin V.I., Kissina L.B., Sidorov K.V. Improving the Technological Plasticity of Steel Kh18N9T. 2nd ed. Moscow: TsIINChM; 1964:10. (In Russ.).
About the Authors
A. A. BabenkoRussian Federation
Anatolii A. Babenko, Dr. Sci. (Eng.), Prof., Chief Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
R. R. Shartdinov
Russian Federation
Ruslan R. Shartdinov, Cand. Sci. (Eng.), Research Associate of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
D. A. Lobanov
Russian Federation
Daniil A. Lobanov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Problems of Man-Made Formations
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. N. Smetannikov
Russian Federation
Artem N. Smetannikov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. G. Upolovnikova
Russian Federation
Alena G. Upolovnikova, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
V. S. Gulyakov
Russian Federation
Vladimir S. Gulyakov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Babenko A.A., Shartdinov R.R., Lobanov D.A., Smetannikov A.N., Upolovnikova A.G., Gulyakov V.S. Features of application of boron-containing slags in stainless steel smelting. Izvestiya. Ferrous Metallurgy. 2025;68(5):488-494. https://doi.org/10.17073/0368-0797-2025-5-488-494