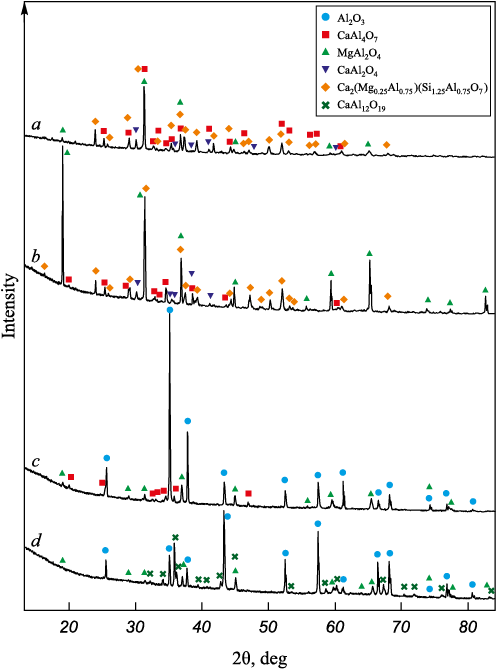Scroll to:
Interaction of Al2O3-based ceramics with slag melt 45 % CaO – 40 % Al2O3 – 10 % SiO2 – 5 % MgO
https://doi.org/10.17073/0368-0797-2025-5-468-475
Abstract
In steelmaking, the refractory material used as a lining is easily destroyed by slag, which not only decreases the service life of ceramics but also makes worse a production quality because of increase in the number of nonmetallic inclusions in metal. If the slag has good wettability, it tends to penetrate the refractory through pores and cracks. As a result, a boundary layer is formed, which has a structure and properties different from the initial material. The sessile drop method was used to study the interaction of Al2O3-based refractory ceramics with the liquid slag 45 % CaO – 40 % Al2O3 – 10 % SiO2 – 5 % MgO. The substantial decrease in the wetting angle to 20° in the initial 5 min of experiment and the further small decrease to 13.5° in the next 115 min were observed. The microstructural examination and elemental mapping of the boundaries of cross sections of slag and ceramics were carried out. The slag consists of several phases, namely: Ca2(Mg0.25Al0.75 )(Si1.25Al0.75O7 ); CaAl2O4 , CaAl4O7 and MgAl2O4 . As was found, the slag–ceramics boundary layer consisted of calcium aluminate (CaAl4O7 ) and, at the grain boundaries of aluminum oxide in the refractory material, hibonite (CaAl12O19 ) was formed. X-ray diffraction analysis of initial ceramics showed that it contained ~8 % CaAl4O7 , and after interaction with the slag ~32 % CaAl12O19 . At a depth of 4 mm, the presence of calcium aluminates both in the central and peripheral zones of ceramics was observed. This indicates the slag penetration into the ceramics and their chemical interaction.
Keywords
For citations:
Aleksandrov A.A., Anuchkin S.N., Kanevskiy A.G., Rumyantseva S.B., Grigorovich K.V. Interaction of Al2O3-based ceramics with slag melt 45 % CaO – 40 % Al2O3 – 10 % SiO2 – 5 % MgO. Izvestiya. Ferrous Metallurgy. 2025;68(5):468-475. https://doi.org/10.17073/0368-0797-2025-5-468-475
Introduction
The increasing requirements for steel quality and the improvement of steel cleanliness through the removal of nonmetallic inclusions are among the main objectives of metallurgy. Nonmetallic inclusions are formed during steel deoxidation, erosion and corrosion of refractories, ingress of slag particles into the melt, and directly during metal solidification. In steelmaking, the refractory used as a lining is easily destroyed by slag, which not only decreases the service life of refractory ceramics but also reduces production quality by increasing the number of nonmetallic inclusions in the metal. Frequent replacement of refractories increases costs and decreases productivity, thus negating the advantages of introducing new methods for liquid steel treatment.
As noted in [1 – 3], the destruction of refractories is most often caused by chemical corrosion and slag penetration. Corrosion damage of refractory ceramics is mainly due to their chemical interaction with slags [4]. The corrosion behavior is strongly influenced by the wettability of refractories by slags [5; 6]. Good wettability indicates active interaction and means that the slag easily reacts with the refractory material, causing its chemical corrosion [6 – 9]. If the slag has good wettability, it tends to penetrate the refractory through pores and cracks [10]. As a result, a boundary layer is formed, with structure and properties different from those of the initial refractory.
Developing refractories resistant to slag attack remains a significant research focus in modern metallurgy [11 – 15]. Thermophysical properties, viscosity, surface tension, and wetting angle are the primary indicators of slag–refractory interaction and penetration. In recent decades, numerous studies of the interaction between refractories and various slags have employed the sessile drop method, in which a slag sample is placed on a refractory ceramic substrate [7 – 9; 16 – 18]. This method makes it possible to analyze the wettability of refractory ceramics by slag melts, to study surface tension and wetting angle as functions of temperature, gas phase, and contact time between the drop and the substrate, and to examine the chemical interaction between the refractory and the slag.
An important aspect of metallurgical production is purging of the melt with inert gases at various stages of steel treatment. During ladle treatment, purging plugs interact both with liquid metal [19] and, after the melt has been poured out of the ladle, with slag. This service environment leads to wear of the plugs; therefore, to study changes in the composition of the plug ceramics, it is necessary to investigate their interaction not only with liquid steel but also with slag. As a rule, a typical ladle slag used in the processing of low-carbon steels (pipe steels, automotive sheet steels, etc.) has the composition: 45 % CaO, 40 % Al2O3 , 10 % SiO2 , 5 % MgO, with basicity CaO/SiO2 = 4.5. The purpose of this study is to investigate the interaction of slag in the CaO – Al2O3 – SiO2 – MgO system with Al2O3-based ceramics, and to analyze changes in the microstructure and macrostructure of the ceramics and the slag as functions of temperature and interaction time.
Materials and methods
The materials investigated were the refractory used to manufacture ladle purging plugs (95.81 % Al2O3 , 2.22 % MgO, 1.35 % CaO, 0.33 % Na2O, 0.06 % SiO2 , 0.02 % Fe2O3 ) and a ladle slag (45 % CaO, 40 % Al2O3 , 10 % SiO2 , 5 % MgO). The slag of this composition was prepared from pre-annealed pure oxides. The oxide powders were first mixed in a vibratory cup mill (IV-1). The obtained mixture was then melted in an Al2O3 crucible placed inside an outer graphite crucible, using an induction furnace powered by a high-frequency generator CEIA Power Cube 180/50 (50 kHz, 180 kVA). After melting, the resulting slag was crushed and pressed into pellets 6 mm in diameter, 6 mm in height, and weighing approximately 0.3 g.
The experiments were conducted in a vacuum resistance furnace equipped with a graphite heater, housing a seamless molybdenum tube. A ceramic substrate (40×30×6 mm) was placed on a stand at the tube center, and a slag pellet was positioned on the substrate surface. An optical system provided magnified imaging, which was recorded with a digital camera for subsequent processing. A detailed description and schematic of the setup are given in [20]. The experiments were performed as follows. The system was evacuated to 5 Pa, after which the slag sample was heated to 1273 K. The test was then carried out in an atmosphere of high-purity Ar. After the slag melted and the temperature reached 1783 – 1793 K, the sample was held isothermally for 2 h. Changes in the slag pellet profile were recorded with a digital camera as functions of holding time and temperature. Upon completion of the experiments, cross sections of the slag and ceramics at the contact area were prepared, and the interaction zone was examined using a scanning electron microscope (SEM) equipped with an electron probe microanalyzer JEOL JXA-iSP100. For X-ray diffraction (XRD) analysis, a Tongda TD-3700 diffractometer with a vertical goniometer and a high-speed Mythen detector was used. The phase composition of the samples was determined with the QualX software package using the PDF2 ICDD database, while the quantitative phase composition was refined in the MAUD software package by the Rietveld method.
Results and discussion
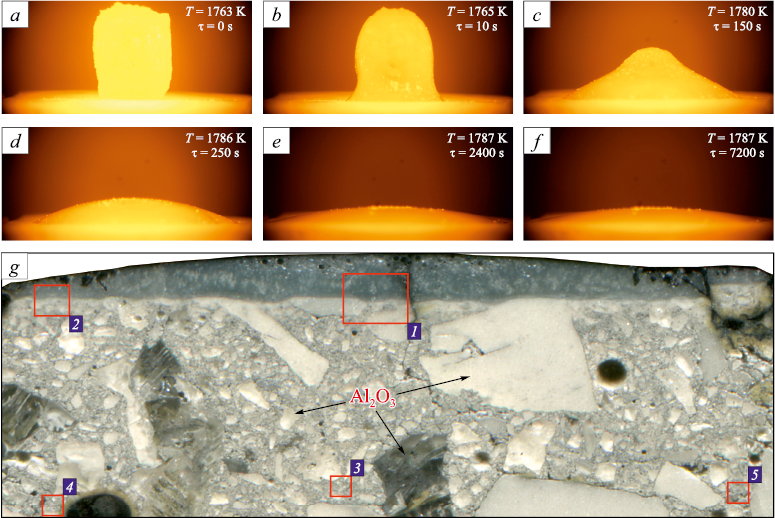
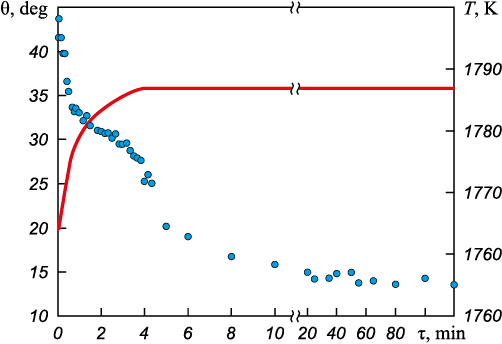
The interaction of slag (45 % CaO, 40 % Al2O3 , 10 % SiO2 , 5 % MgO) with aluminum oxide-based refractory ceramics was studied. Fig. 1 shows the change in the slag sample profile depending on temperature and holding time. From the recorded images of the slag drop profile, the wetting angle (Fig. 2) was calculated as the mean value between the right and left contact angles. Notably, the starting point of measurement corresponded to the onset of slag tablet melting (Fig. 1, a), whereas the formation of a completely liquid slag drop (Fig. 1, d) occurred only after 220 s. Analysis of the wetting angle revealed a sharp decrease during the first 5 min of the test and a short plateau lasting 1.5 – 3.0 min (31.0 – 29.5°), probably associated with the melting process. After complete formation of the drop, the wetting angle (θ) decreased from 28 to 20° within 1.5 min (up to 5 min of the test), followed by a gradual decrease to 13.5° over 115 min. Fig. 2 shows the variation in the wetting angle with temperature and holding time. During the test, partial penetration of slag into the ceramics was observed (Figs. 1, e – f). These findings demonstrate good wettability of the refractory ceramics by the slag, which can subsequently lead to erosion and chemical corrosion, ultimately reducing the service life of the refractory [5; 21; 22]. Therefore, the microstructure of the slag and ceramics after the test was examined in more detail.
Fig. 1. Slag sample profile depending on holding time and temperature (а – f);
Fig. 2. Wetting angle depending on temperature and holding time: |
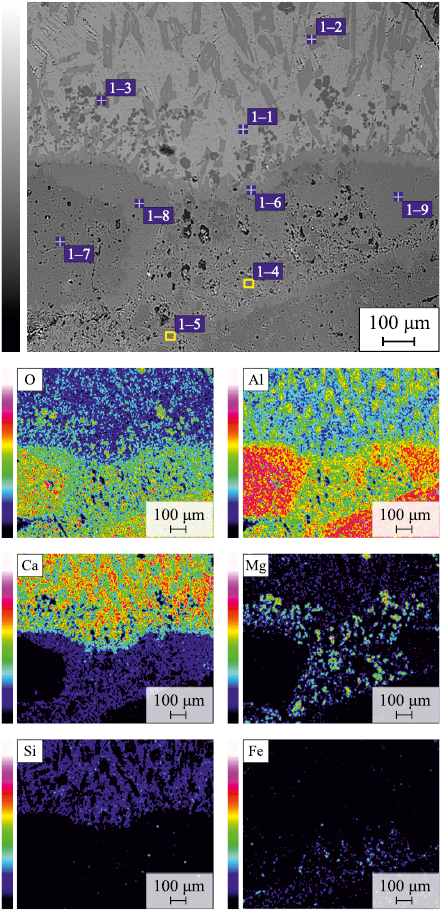
Fig. 1 shows a cross section of the ceramic substrate after the experiment, indicating the zones of slag and ceramics where the analyses were performed. Using a scanning electron microscope JEOL JXA-iSP100, the slag–ceramics interaction zones (zones 1 and 2 in Fig. 1) and various zones of the ceramics (zones 3 – 5 in Fig. 1) were examined.
Fig. 3 presents the microstructure and elemental mapping of the slag–ceramics boundary layer (zone 1 in Fig. 1). The slag consists of several structural zones: light-gray, gray, and dark-gray. In the light-gray zone, the presence of calcium, silicon, aluminum, and magnesium was observed (spectrum 1–1 in Fig. 3). The elemental compositions of the spectra are given in the Table. The gray zone contains aluminum and calcium (spectrum 1–2 in Fig. 3), whereas the dark-gray zone is rich in magnesium and aluminum (spectrum 1–3 in Fig. 3). XRD analysis of the initial slag (Fig. 4, a) and the slag after interaction with ceramics (Fig. 4, b) showed that it consists of four phases: Ca2(Mg0.25Al0.75 )(Si1.25Al0.75O7 ), CaAl2O4 , CaAl4O7 , and MgAl2O4 . Based on the elemental analysis, it can be assumed that the light-gray zone corresponds to Ca2(Mg0.25Al0.75)(Si1.25Al0.75O7 ), the dark-gray zone to MgAl2O4 , and the gray zone to calcium aluminates [23]. The quantitative phase ratio changed only slightly before and after the experiment: approximately 62 and 57 % for Ca2(Mg0.25Al0.75 )(Si1.25Al0.75O7 ), 16 and 15 % for CaAl2O4 , 10 and 14 % for CaAl4O7 , and 14 and 14 % for MgAl2O4 , respectively.
Fig. 3. Microstructure (backscattered electron mode)
Elemental composition of the slag and ceramics represented in Figs. 4 and 5
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Elemental analysis and mapping of the ceramics interaction zone (Fig. 3) revealed possible diffusion of magnesium and calcium from the slag into the ceramics (spectra 1–4 and 1–5 in Fig. 3). The boundary layer consists of calcium aluminate (spectrum 1–6 in Fig. 3), which, according to the phase diagram [23], corresponds to CaAl4O7 . At the grain boundaries of aluminum oxide (spectrum 1–7 in Fig. 3), a phase containing calcium and aluminum (spectrum 1–8 in Fig. 3) was detected, corresponding to hibonite (CaAl12O19 ). Small aluminum oxide grains were found to have completely transformed into hibonite (spectrum 1–9 in Fig. 3. XRD analysis of the initial ceramics (Fig. 4, c) showed that it contained approximately 82 % Al2O3 , 10 % MgAl2O4 , and 8 % CaAl4O7 . After interaction with the slag (Fig. 4, d), the ceramics consisted of about 56 % Al2O3 , 12 % MgAl2O4 , and 32 % CaAl12O19 . These results indicate penetration of slag into the ceramics and its chemical interaction with the material, accompanied by the formation of calcium aluminates.
Fig. 4. XRD spectra of the initial slag (а), slag after its interaction |
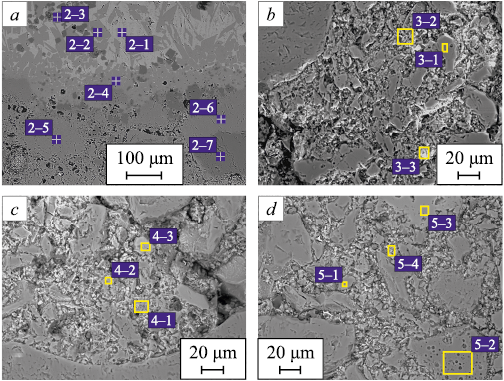
Fig. 5 shows the microstructures of the slag–ceramics interaction zone (zone 2 in Fig. 1) and various zones of the ceramics (zones 3 – 5 in Fig. 1). Elemental analysis of the slag–ceramics interaction zone (Fig. 5, a) demonstrates that (1) the slag phase also consists of three structural zones (spectra 2–1 to 2–3 in Fig. 5, a); (2) the interfacial zone is composed of calcium aluminate (spectrum 2–4 in Fig. 5, a); and (3) as in the central interaction zone, complete transformation of small aluminum oxide grains into hibonite (CaAl12O19 ) (spectrum 2–5 in Fig. 5, a) and its formation along the boundaries of larger grains were observed (spectra 2–6 and 2–7 in Fig. 5, a).
Fig. 5. Microstructure (backscattered electron mode) |
Elemental analysis of the central zone of the ceramics at a depth of about 4 mm (Fig. 5, b) revealed both the alumomagnesium component of the ceramics (spectrum 3–1 in Fig. 5, b) and an increased calcium content (spectra 3–2 and 3–3 in Fig. 5, b). This confirms slag penetration into the ceramic substrate during the experiment. Figs. 5, c and d show the edge zones of the ceramics at a depth of approximately 4 mm. These zones contain the alumomagnesium component (spectrum 5–1 in Fig. 5, d) and aluminum oxide grains (spectrum 5–2 in Fig. 5, d), as well as areas with elevated calcium content (spectra 4–1 to 4–3, 5–3, and 5–4 in Figs. 5, c, d), corresponding in composition to phases close to calcium aluminates CaAl4O7 and CaAl12O19 .
Thus, it can be concluded that during the experiment, chemical interaction of the slag with the ceramics occurred, resulting in the formation of hibonite (CaAl12O19 ) within both small and large aluminum oxide grains, accompanied by noticeable slag penetration into the ceramic substrate.
Conclusions
The interaction of aluminum oxide-based refractory ceramics, used in the manufacture of purging plugs, with ladle slag of composition 45 % CaO, 40 % Al2O3 , 10 % SiO2 , and 5 % MgO was investigated. A substantial decrease in the wetting angle (θ) to 20° was observed during the first 5 min of the test, followed by a slight further decrease to 13.5° over 115 min. This indicates good wettability of aluminum oxide-based refractory ceramics by the slag.
Microstructural examination and elemental mapping of the cross-sectional slag–ceramics boundary layer revealed that the slag consists of several structural zones: light-gray, gray, and dark-gray. According to XRD analysis, the light-gray zone corresponds to the compound Ca2(Mg0.25Al0.75)(Si1.25Al0.75O7 ), the dark-gray region to MgAl2O4, and the gray region to calcium aluminates. Only minor changes in the phase ratios were observed before and after the experiment.
Analysis of the interaction zone showed possible diffusion of magnesium and calcium from the slag into the ceramics. The slag–ceramics boundary layer was found to consist of calcium aluminate (CaAl4O7 ). At the grain boundaries of aluminum oxide, the formation of a phase corresponding to hibonite (CaAl12O19 ) was observed. Small aluminum oxide grains were completely transformed into hibonite. XRD analysis of the initial ceramics showed that it contained approximately 8 % CaAl4O7 , while the ceramics after interaction with slag contained about 32 % CaAl12O19 . This confirms slag penetration into the ceramics and its chemical interaction with the material accompanied by the formation of calcium aluminates.
Elemental analysis of the ceramics at a depth of about 4 mm revealed the presence of calcium aluminates (close in composition to CaAl4O7 and CaAl12O19 ) in both the central and peripheral zones. This indicates slag penetration into the ceramic substrate during the experiment.
References
1. Han J.S., Kang J.G., Shin J.H., Chung Y., Park J.H. Influence of CaF2 in calcium aluminate-based slag on the degradation of magnesia refractory. Ceramics International. 2018;44(11):13197–13204. https://doi.org/10.1016/j.ceramint.2018.04.145
2. Ren X.M., Ma B.Y., Li S.M., Li H.X., Liu G.Q., Yang W.G., Qian F., Zhao S.X., Yu J.K. Comparison study of slag corrosion resistance of MgO–MgAl2O4 , MgO–CaO and MgO–C refractories under electromagnetic field. Journal of Iron Steel Research International. 2021;28(1):38–45. https://doi.org/10.1007/s42243-020-00421-0
3. Yan Z., Deng Z., Zhu M. Penetration behavior of CaO–SiO2–FeOx–MgO–(CaCl2) slags in MgO refractory. Metallurgical and Materials Transactions B. 2023;54(3):1582–1592. https://doi.org/10.1007/s11663-023-02787-4
4. Park J.H., Suk M.O., Jung I.H., Guo M., Blanpain B. Interfacial reaction between refractory materials and metallurgical slags containing fluoride. Steel Research International. 2010; 81(10):860–868. https://doi.org/10.1002/srin.201000157
5. Wang Y.-x., Li Y.-g., Gao Y.-b., Huang Zh., Zhang H.-j. High-temperature wetting behavior between slag and refractory. Journal of Iron Steel Research International. 2024;31: 1304–1319. https://doi.org/10.1007/s42243-024-01252-z
6. Gehre P., Aneziris C.G., Berek H., Parr C., Reinmöller M. Corrosion of magnesium aluminate spinel-rich refractories by sulphur-containing slag. Journal of the European Ceramic Society. 2015;35(5):1613–1620. https://doi.org/10.1016/j.jeurceramsoc.2014.11.031
7. Park J., Lee K., Pak J.J., Chung Y. Initial wetting and spreading phenomena of a CaO–SiO2 liquid slag on MgO substrates. ISIJ International. 2014;54(9):2059–2063. https://doi.org/10.2355/isijinternational.54.2059
8. Yu B., Lv X., Xiang S., Bai C., Yin J. Wetting behavior of calcium ferrite melts on sintered MgO. ISIJ International. 2015;55(8):1558–1564. https://doi.org/10.2355/isijinternational.ISIJINT-2014-830
9. Park J., Jeon J., Lee K., Park J.H., Chung Y. Initial wetting and spreading rates between SiC and CaO–SiO2–MnO slag. Metallurgical and Materials Transactions B. 2016;47(3): 1832–1838. https://doi.org/10.1007/s11663-016-0606-0
10. Huang F., Liu C., Maruoka N., Kitamura S.Y. Dissolution behaviour of MgO based refractories in CaO–Al2O3–SiO2 slag. Ironmaking & Steelmaking. 2015;42(7):553–560. https://doi.org/10.1179/1743281215Y.0000000003
11. Zou Y., Huang A., Wang R., Fu L., Gu H., Li G. Slag corrosion-resistance mechanism of lightweight magnesia-based refractories under a static magnetic field. Corrosion Science. 2020; 167:108517. https://doi.org/10.1016/j.corsci.2020.108517
12. Fu L., Huang A., Lian P., Gu H. Isolation or corrosion of microporous alumina in contact with various CaO–Al2O3–SiO2 slags. Corrosion Science. 2017;120:211–218. https://doi.org/10.1016/j.corsci.2017.01.018
13. Ren X., Ma B., Li S., Li H., Liu G., Zhao S., Yang W., Qian F., Yu J. Slag corrosion characteristics of MgO-based refractories under vacuum electromagnetic field. Journal of the Australian Ceramic Society. 2019;55:913–920. https://doi.org/10.1007/s41779-019-00323-9
14. Ma B., Yin Y., Zhu Q., Zhai Y., Li Y., Li G., Yu J. Slag corrosion and penetration behaviors of MgAl2O4 and Al2O3 based refractories. Refractories and Industrial Ceramics. 2016;56(5): 494–501. https://doi.org/10.1007/s11148-016-9876-y
15. Vázquez B.A., Pena P., De Aza A.H., Sainz M.A., Caballero A. Corrosion mechanism of polycrystalline corundum and calcium hexaluminate by calcium silicate slags. Journal of the European Ceramic Society. 2009;29(8):1347–1360. https://doi.org/10.1016/j.jeurceramsoc.2008.08.031
16. Song J., Liu Y., Lv X., You Z. Corrosion behavior of Al2O3 substrate by SiO2–MgO–FeO–CaO–Al2O3 slag. Journal of Materials Research and Technology. 2020;9(1):314–321. https://doi.org/10.1016/j.jmrt.2019.10.060
17. Shen P., Zhang L., Wang Y., Sridhar S., Wang Q. Wettability between molten slag and dolomitic refractory. Ceramics International. 2016;42(14):16040–16048. https://doi.org/10.1016/j.ceramint.2016.07.113
18. Wang C., Xie C., Xu J., Wan K. Wettability and spreading kinetics between some refractory materials and molten calcium aluminate slag. Steel Research International. 2025;(6): 2400895. https://doi.org/10.1002/srin.202400895
19. Shvarts K., Kraus O. Study of penetration of liquid metal into purge plugs. Ogneupory i tekhnicheskaya keramika. 2013;(4-5):52–56. (In Russ.).
20. Ahuchkin S.N., Burtsev V.T., Zagumennikov M.V., Sidorov V.V., Rigin V.E. Study of the surface properties of nickel-based melts by the constrained drop method: I. Surface tension. Russian Metallurgy (Metally). 2010;(1):13–17. https://doi.org/10.1134/S0036029510010039
21. Yang M., Yan Z., Li Z., Lv X., Bai C. Modelling the dissolutive wetting of slag-oxide system at high temperatures. Metallurgical and Materials Transactions B. 2025;56(2): 1573–1587. https://doi.org/10.1007/s11663-024-03429-z
22. Monaghan B.J., Abdeyazdan H., Dogan N., Rhamdhani M.A., Longbottom R.J., Chapman M.W. Effect of slag composition on wettability of oxide inclusions. ISIJ International. 2015;55(9):1834–1840. https://doi.org/10.2355/isijinternational.ISIJINT-2015-168
23. Slag Atlas: Handbook. Düsseldorf: Verlag Stahleisen GmbH; 1995:634.
About the Authors
A. A. AleksandrovRussian Federation
Aleksandr A. Aleksandrov, Cand. Sci. (Eng.), Senior Researcher, Head of the A.M. Samarin Laboratory of Physical Chemistry of Metal Melts
49 Leninskii Ave., Moscow 119334, Russian Federation
S. N. Anuchkin
Russian Federation
Sergei N. Anuchkin, Cand. Sci. (Eng.), Senior Researcher of the A.M. Samarin Laboratory of Physical Chemistry of Metal Melts
49 Leninskii Ave., Moscow 119334, Russian Federation
A. G. Kanevskiy
Russian Federation
Akim G. Kanevskiy, Cand. Sci. (Eng.), Senior Researcher of the A.M. Samarin Laboratory of Physical Chemistry of Metal Melts
49 Leninskii Ave., Moscow 119334, Russian Federation
S. B. Rumyantseva
Russian Federation
Sof’ya B. Rumyantseva, Cand. Sci. (Eng.), Research Associate of the Laboratory of Materials Diagnostics
49 Leninskii Ave., Moscow 119334, Russian Federation
K. V. Grigorovich
Russian Federation
Konstantin V. Grigorovich, Academician, Dr. Sci. (Eng.), Head of the Laboratory of Materials Diagnostics, Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences; Prof. of the Chair of Metallurgy of Steel, New Production Technologies and Metal Protection, National University of Science and Technology “MISIS”
49 Leninskii Ave., Moscow 119334, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
Review
For citations:
Aleksandrov A.A., Anuchkin S.N., Kanevskiy A.G., Rumyantseva S.B., Grigorovich K.V. Interaction of Al2O3-based ceramics with slag melt 45 % CaO – 40 % Al2O3 – 10 % SiO2 – 5 % MgO. Izvestiya. Ferrous Metallurgy. 2025;68(5):468-475. https://doi.org/10.17073/0368-0797-2025-5-468-475