Scroll to:
Carburization of pellets to a carbon content of more than 4.5 % during metallization in shaft furnaces
https://doi.org/10.17073/0368-0797-2025-5-461-467
Abstract
Hot Briquetted Iron (HBI) or Direct Reduced Iron (Pellets) (DRI) is one of the most sought-after products in the steel industry because its use enables the environmentally friendly production of high grade steels. The purpose of this paper is to study the process of pellets carburization under the conditions of a shaft direct reduction (metallization) furnace in comparison with the carburization of pellets due to the preparation of an ore-carbon burden. Hot briquetted iron produced in the HYL-III process is different from Midrex briquettes in terms of carbon content. Difference in the amount of carbon is attributed to the processes of carburization and pyrolysis of natural gas in the shaft furnace workspace, as well as difference in composition of the gas phase and pressure in the workspace of the HYL and Midrex furnaces. As is known, the HYL-III process utilizes vapor conversion (higher H2/CO ratio) at elevated gas pressures beneath the furnace top, in contrast to the Midrex process. An increase in the carbon monoxide (CO) content in the gas phase of the Midrex process (carbon dioxide conversion) results in intensification on the pellet surface that was reduced to metal. The findings of the study demonstrated that carburization of pellets to a greater than 4.5 % carbon content through the process of gas metallization (direct reduction) in shaft furnaces is indeed feasible. The Midrex process, which relies on the reducing agent, mostly carbon monoxide (CO), allows for the treatment of pellets with methane. In contrast, the HYL process, which utilizes hydrogen (H2 ) mostly as the reducing agent, necessitates the addition of solid carbon, such as soot or coke breeze etc., to the burden for carburization. This finding suggests the potential for utilization of carbon-containing briquettes in metallization processes. Carbon, despite its presence in the form of a separate phase (soot), cannot be separated from the iron-containing components of pellets by magnetic separation or washing and does not pose any danger.
For citations:
Sabirov E.R., Pokolenko A.Yu., Bizhanov A.M., Bersenev I.S., Spirin N.A. Carburization of pellets to a carbon content of more than 4.5 % during metallization in shaft furnaces. Izvestiya. Ferrous Metallurgy. 2025;68(5):461-467. https://doi.org/10.17073/0368-0797-2025-5-461-467
Introduction and problem statement
Hot Briquetted Iron (HBI) or direct reduced pellets (DRI) are among the most sought-after products in the steel industry, since their use enables the environmentally friendly production of high-grade steels [1 – 4]. This, in particular, explains the increasing production of direct reduced iron1. One of the important quality parameters of this product is the carbon content, which led to the development of the ACT Midrex technology2. According to the developer, this technology makes it possible to achieve a carbon content in metallized pellets of up to 4.5 wt. %. Assessing the conditions required to achieve this level and comparing them with the alternative approaches is an important objective, as it expands the tools available for improving the metallurgical properties of HBI. One of the factors determining the quality of the metallized product (including the carbon content) is the material composition of pellets [5]. This aspect is not analyzed in the present study (the raw material in all the samples is identical), and the investigation focuses solely on the kinetics of carburization. The basis for the study was the primary data reported in [5; 6], along with additional experiments. Table 1 shows the distribution of carbon in HBI produced at Lebedinsky Mining and Processing Plant (Lebedinsky GOK) across three technological lines.
Table 1. Average carbon content in HBI [6]
| ||||||||||||
According to these data, HBI produced by the HYL-III process differs in carbon content from Midrex briquettes. The difference in carbon amount between the samples is attributed to the processes of carburization and natural gas pyrolysis in the shaft furnace workspace, as well as to differences in the gas phase composition and pressure in the furnace workspace of the HYL and Midrex processes [7 – 9]. It is well known, the HYL-III process employs vapor conversion (higher H2/CO ratio) at higher gas pressures beneath the furnace top compared to Midrex. The higher CO content in the gas phase of the Midrex process (carbon dioxide conversion) intensifies the reaction on the metallic surface of the pellet [10]:
| Fe + 6CO → Fe3C + 3CO2 . | (1) |
Carbon in pellets is distributed between iron carbides and a separate phase – soot. The purpose of this study is to investigate the process of carburization of pellets (to a carbon content >4.5 %) in a shaft furnace, in comparison with carburization of pellets through the formation of an ore–carbon burden.
Investigation of gas-phase carburization
of direct reduced pellets
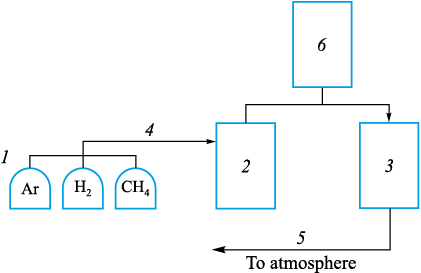
To determine the conditions influencing the carburization of pellets, a research stand was prepared (Fig. 1). It consisted of gas cylinders (1), a vertical electric furnace containing a reaction crucible (2), a combustion chamber (3), gas supply (4) and exhaust systems (5) with filters, and a gas analyzer (6).
Fig. 1. Diagram of the research stand |
Methane was fed into the reaction crucible and heated as it passed between the walls of the outer and inner tubes, flowing downward. It then moved upward through the perforated bottom (ceramic beads) of the inner tube and was discharged from the crucible. It then entered a sealed vessel filled with water, which served both as a filter and as a cooler to cool the gas before its delivery to the analyzer. Downstream of the analyzer, the gas was routed to the combustion chamber. This chamber was used to neutralize explosive components formed during methane cracking, preventing their accumulation and potential ignition in the laboratory. The temperature and gas flow rate were kept constant during the isothermal holding stage. During the test, elapsed time, mass, temperature, inlet-gas flow rate, and outlet-gas composition were recorded and logged. Methane decomposition was monitored by changes in retort mass and by analyzing the chemical composition of the outlet gas. Methane (CH4 – 99.99 %; balance CO, CO2 , N2 , H2O, O2 , CmHn ) was supplied from a cylinder, and argon (Ar – 99.993 %) was used as the inert gas.
Experiments were conducted to simulate the carburization zone of a shaft furnace using direct reduced pellets (degree of reduction 95 %). The tests were carried out at temperatures of 1000 and 1100 °C (Table 2). The methane flow rate was determined from the reaction zone volume, the gas tract capacity of the unit, and the need to maintain a stable regulation range. The data in Table 2 show that the presence of direct reduced pellets resulted in an increase in pellet mass due to soot deposition.
Table 2. Experimental conditions and formation
|
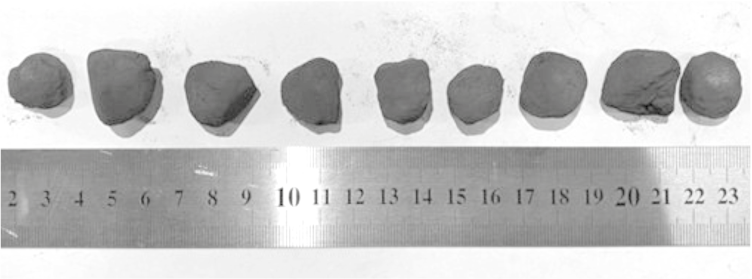
At a methane flow rate of 1.757 L/min, the maximum increase in pellet mass due to carbon deposition was 56.48 g/h, with most of the carbon depositing on the surface of the metallized pellets and within their pores. As a result, upon completion of the tests, the samples grew in size (from 12 to 20 – 22 mm), and their shape changed from spherical to angular (Fig. 2). Phase analysis revealed up to 5 wt. % total carbon and more than 2 wt. % carbon in the form of soot.
Fig. 2. View of pellets after reduction |
To determine the possibility of separating the soot carbon from the metal, the pellets were ground to a –100 µm fraction. When the dry fine material was separated by a magnet into magnetic and non-magnetic fractions, it was found that all of the material was magnetic. Washing the ground material in water also failed to separate the phases. Therefore, isolating carbon as a separate product by these methods proved impossible.
Investigation of solid-carbon carburization
of direct reduced pellets
Pellets and briquettes with an organic binder were prepared for the laboratory tests. The organic binder does not reduce the content of valuable components in the metallized product. It promotes the development of a microporous structure during reduction – heat treatment and ensures adequate strength of the products at intermediate production stages (green handling, drying). Limestone from the Lebedinsky GOK was used as the flux. The chemical composition of the components is given in Table 3. The soot contained 99.59 % carbon. The particle-size distribution of the components met the requirements for pellet production. The charge for briquettes and pellets consisted of 89.62 % concentrate, 5.35 % soot, 2.03 % limestone, and 3 % organic binder (dry basis).
Table 3. Chemical composition of burden components
| |||||||||||||||||||||||||||||||||||||||
A laboratory stand (Fig. 1) was used to carry out the reduction–heat treatment of ore–carbon samples. The heat treatment was carried out as follows. The samples were heated together with the furnace to 1000 °C in an inert atmosphere (argon remained in the retort after leak-tightness testing). During heating, iron oxides were reduced by the solid reductant (soot) present in the samples, as no air was supplied to the retort. The smoke generated during carbon reduction was vented to atmosphere. When the sample mass reached a constant value and smoke emission from the retort ceased, it was it was considered that the carbon had fully burned out. Hydrogen was then introduced into the reaction crucible and the reduction of the pellets continued to a degree of reduction >90 %. When constant mass was attained, the hydrogen supply to the retort was stopped and replaced with inert gas. The retort was removed from the furnace, and the inert gas continued to be supplied until the temperature fell to 60 °C. The retort was then disassembled and the sample taken out.
The experiments showed that the surfaces of the dried pellets tended to crumble during loading and transfer. Because the pellets were not coated with chalk or cement suspensions to suppress sintering, sintering occurred during reduction. For the reduced samples (Fig. 3), we determined compressive strength (minimum requirement ≥30 kg/pellet) and the chemical composition of the target components (Table 4). In addition, tests were performed on samples reduced solely by soot – i.e., without hydrogen supply to the retort – to assess the effect of carbon on oxygen removal from the iron-ore particles.
Fig. 3. Typical appearance of the reduced samples of pellets (a, b) and briquettes (c, d),
Table 4. Physico-chemical properties of the reduced samples
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The data indicate that the compressive strength of pellets reduced solely by soot differed by no more than ~3 % (relative). Accordingly, carbon-containing briquettes can be used in metallization without compromising the strength of the reduced products, as also suggested in [11; 12]. The compressive strength of pellets reduced by soot was 1.5 – 2.0 times lower than that of pellets reduced by hydrogen. This is attributed to the presence of different phases in the samples characteristic of a metallization degree of ~45 %: Fe3O4 , FeO, and Femet . In contrast, when the pellets had a homogeneous structure represented by metallic iron, their strength increased. This agrees with the results reported in [13; 14]. The compressive strength of briquettes likewise increased with metallization degree, mirroring the trend observed for pellets. Reduction of ore–carbon samples solely by soot (no H2 feed) yielded a degree of reduction of ~45 – 50 %. At this stage, extensive reaction surfaces developed owing to burnout of the organic binder and carbon, as well as the magnetite → wüstite phase transition.
Conclusions
The experiments demonstrate that achieving >4.5 wt. % carbon in pellets by gas-phase metallization in shaft furnaces is feasible. In the Midrex process (reducing agent predominantly CO), this can be attained by methane treatment of pellets; in the HYL process (reducing agent predominantly H2 ), carburization requires adding solid carbon (soot, coke breeze, etc.) to the burden. Although present as a separate phase (soot), carbon cannot be separated from the iron-bearing pellet components by magnetic separation or washing and poses no hazard. During reduction and carburization of pellets and briquettes, deformation occurred, accompanied by volumetric expansion and crack formation.
References
1. Petrov S.P. Ferrous Metallurgy in Asian Russia in the Second and Third Decades of the 21st Century. Novosibirsk: Institute of Economics and Industrial Organization of the Siberian Branch of RAS; 2023:240. (In Russ.).
2. Budanov I.A. Macroeconomic prospects of steel metal production. Stal’. 2024;(6):47–53. (In Russ.).
3. Bilici S., Holtz G., Jülich A., etc. Global trade of green iron as a game changer for a near-zero global steel industry? A scenario-based assessment of regionalized impacts. Energy and Climate Change. 2024;5:100161. https://doi.org/10.1016/j.egycc.2024.100161
4. Anderson S.H. HBI improves eat energy efficiency and yield and downstream operating results: Educated use of DRI. Charlotte, NC, USA: MIDREX Technologies Inc.; 2001:1–11.
5. Bersenev I.S., Vokhmyakova I.S., Borodin A.V., etc. Prediction of the quality of hot briquetted iron (HBI) based on data on the material composition of pellets. Steel in Translation. 2022;52(7):673–676. https://doi.org/10.3103/S0967091222070038
6. Vokhmyakov I.S., Bersenev I.S., Borodin A.V., Sivkov O.G., Stepanova A.A., Kirienkov A.N. Mechanism of oxidation for hot briquetting iron (HBI). Steel in Translation. 2022;52(3):331–336. https://doi.org/10.3103/S0967091222030160
7. Kumar T.K.S., Alatalo J., Ahmed H., etc. Effect of temperature and gas mixtures on cementite formation during the carburization of hydrogen-reduced DRI. Journal of Sustainable Metallurgy. 2022;8:1450–1464. https://doi.org/10.1007/s40831-022-00601-0
8. Perrone A., Cavaliere P., Sadeghi B., Dijon L., Laska A., Koszelow D. Carburization behavior of high-grade pellets after direct reduction in pure hydrogen. Journal of Sustainable Metallurgy. 2024;10:1991–2008. https://doi.org/10.1007/s40831-024-00906-2
9. Dishwar R.K., Mandal A.K., Sinha O.P. Studies on reduction behaviour of highly fluxed iron ore pellets for application in steelmaking. Materials Today: Proceedings. 2021;46(3): 1471–1475. https://doi.org/10.1016/j.matpr.2020.10.886
10. Bogdandy L., Engell H.-J. Die Reduktion der Eisenerze. Düsseldorf: Springer Verlag; 1967:539. (In Germ.).
11. Lohmeier L., Thaler C., Harris C., Wollenberg R., Schröder H.-W., Braeuer A.S. Use of bentonite and organic binders in the briquetting of particulate residues from the Midrex process for improving the thermal stability and reducibility of the briquettes. Steel Research International. 2021;92(12):2100210. https://doi.org/10.1002/srin.202100210
12. Mizutani M., Nishimura T., Orimoto T., Higuchi K., Nomura S., Saito K., Kasai E. Influence of reducing gas composition on disintegration behavior of iron ore agglomerates. ISIJ International. 2017;57(9):1499–1508. https://doi.org/10.2355/isijinternational.ISIJINT-2017-074
13. Dwarapudi S., Sekhar C., Paul I., Modi K., Pal A.R., Chakraborty U., Das B.K. Effect of fluxing agents on the quality and microstructure of hematite pellets. International Journal of Metallurgical Engineering. 2017;6(1):18–30.
14. Kurunov I.F., Bizhanov A.M., Wakeel A.K., Mishra B. Behavior of extrusion briquettes in Midrex reactors. Part 2. Metallurgist. 2016;60(3-4):243–247. https://doi.org/10.1007/s11015-016-0281-z
About the Authors
E. R. SabirovRussian Federation
Emil’ R. Sabirov, Senior Engineer, LLC “NPVP TOREKS”; Postgraduate, Ural Federal University named after the first President of Russia B.N. Yeltsin
72 Starozhilov Str., Yekaterinburg 620902, Russian Federation
19 Mira Str., Yekaterinburg 620002, Russian Federation
A. Yu. Pokolenko
Russian Federation
Aleksei Yu. Pokolenko, Head of Advanced Technologies Group
72 Starozhilov Str., Yekaterinburg 620902, Russian Federation
A. M. Bizhanov
Russian Federation
Aitber M. Bizhanov, Cand. Sci. (Eng.), Leading Expert of the Chair of Functional Nanosystems and High-Temperature Materials
4 Leninskii Ave., Moscow 119049, Russian Federation
I. S. Bersenev
Russian Federation
Ivan S. Bersenev, Cand. Sci. (Eng.), Head of the Scientific and Analytical Department, LLC “NPVP TOREKS”; Assist. Prof. of the Chair of Metallurgy of Iron and Alloys, Ural Federal University named after the first President of Russia B.N. Yeltsin
72 Starozhilov Str., Yekaterinburg 620902, Russian Federation
19 Mira Str., Yekaterinburg 620002, Russian Federation
N. A. Spirin
Russian Federation
Nikolai A. Spirin, Dr. Sci. (Eng.), Prof., Head of the Chair “Thermal Physics and Informatics in Metallurgy”
19 Mira Str., Yekaterinburg 620002, Russian Federation
Review
For citations:
Sabirov E.R., Pokolenko A.Yu., Bizhanov A.M., Bersenev I.S., Spirin N.A. Carburization of pellets to a carbon content of more than 4.5 % during metallization in shaft furnaces. Izvestiya. Ferrous Metallurgy. 2025;68(5):461-467. https://doi.org/10.17073/0368-0797-2025-5-461-467