Scroll to:
Metallization of ore-coal briquettes in an annular furnace heated by generator gas
https://doi.org/10.17073/0368-0797-2025-4-383-394
Abstract
The paper considers the features of producing granular pig iron using an annular furnace with a rotating hearth during implementation of ITmk3 technology (Ironmaking Technology Mark Three). The design of a coal gasifier with a synthesized gas purification system and the cross-section of an annular furnace are shown. The article briefly describes the process of industrial production of granular high-quality pig iron. The prospects of using the technology in question on the territory of the Russian Federation were assessed. At the first stage of research on the metallization of iron-ore concentrate (IOC) with coal, the thermogravimetric method of a complete factor experiment was used to determine the optimal metallization conditions. In the experiments, the ratio of IOC was varied: coal, size of coal, lime additives as a percentage of the amount (IOC + coal). As a result of thermogravimetric analysis, the authors obtained the curves of changes in mass of the samples, composition and amount of released gas when the heating temperature changed during sintering of IOC with coal and lime. At the second stage, a laboratory chamber furnace with a portable hearth, heated by generator gas from coal, was developed to test the ITmk3 technology. Ore-coal briquettes were made with a ratio of IOC, coal, bentonite 80:20:5 and heat-treated in a chamber furnace with heating by generator gas from a coal gasifier. Iron-ore concentrate from the Korshunovsky MPP and Kasyanovsky coal from the Cheremkhovsky deposit were used as experimental raw materials. Based on laboratory studies, the authors determined the temperature-time firing mode of ore-coal briquettes, which ensures a high degree of metallization of iron-ore materials of 80 – 87 % when firing briquettes in the temperature range of 1080 – 1424 °C for 40 min. The yield of briquettes after drying and firing was determined to be 66.45 %. The mechanism of solid-phase reduction of iron-ore materials in annular furnaces with a rotating hearth and liquid-phase separation of reduction products is considered. The composition of the gases released during calcination of ore and coal briquettes was determined.
Keywords
For citations:
Kulikov B.P., Storozhev Yu.I., Potapenko A.S. Metallization of ore-coal briquettes in an annular furnace heated by generator gas. Izvestiya. Ferrous Metallurgy. 2025;68(4):383-394. https://doi.org/10.17073/0368-0797-2025-4-383-394
Introduction
Over the past 10 years, the production of direct reduced iron (DRI) has increased by 18 % globally and by 66 % in Russia, indicating rapid development in this sector [1]. The widespread adoption of the direct reduction process was first observed in the 1980s, when natural gas began to be used extensively as a reductant in the mining and metallurgical industry. In addition to natural gas, the use of coal gasification products also proved feasible in the direct reduction of iron. Neither method requires the use of expensive coke [2].
According to metallurgical thermal engineers, the most advantageous option is to produce partially metallized iron-ore materials with a metallization degree of 30 – 50 %, which are used in blast furnace production. Highly metallized iron-ore products with a metallization degree of 85 – 95 % are used for remelting in steelmaking units to obtain high-quality steel [2]. The method of direct reduction of iron-ore materials is particularly suitable for regions that lack natural gas reserves but possess abundant coal deposits.
In the 2000s, more than a dozen inventions were developed in Russia related to the production of granular pig iron using annular furnaces with a rotating hearth, incorporating elements of well-known processes such as FASTMET and ITmk3. One invention [3] describes a method for producing granular pig iron by dosing the components of the iron-ore burden to achieve CaO/MgO and SiO2/Al2O3 ratios within the ranges of 2 – 5 and 4 – 6, respectively, ensuring that the primary slag melting temperature does not exceed 1400 °C. Several other patents [4 – 6] focus on the design features of annular furnaces that allow for optimization of heat exchange processes. In particular, patent [6] describes air-cooled suspended screens with vertical movement capability in the briquette loading and unloading zones, and the loading zone is additionally equipped with a device for feeding protective material onto the hearth.
For Siberian conditions, developments using coal gasifiers [7; 8] are especially attractive, as they enable annular furnaces to operate on gasified fuel in the absence of natural gas. Based on these inventions, a process line was developed for producing a metallized product in an annular furnace with a rotating hearth, allowing for maximum utilization of the heat from exhaust gases [9; 10].
One example of such implementation is the construction of a plant for producing granular pig iron in Cheremkhovo, Irkutsk Region, using ITmk3 technology. The site was selected based on the following factors:
– proximity to one of the main raw material sources – coal (Cheremkhovsky coal basin);
– relatively close location of other key raw material source – iron-ore concentrate (Korshunovsky MPP, Zheleznogorsk, Irkutsk Region);
– a well-developed regional transportation network, including both rail (Trans-Siberian Railway) and road access (federal highway).
The project investor is NPO Khimiko-Metallurgicheskaya Kompaniya LLC. The plant’s design capacity is 100,000 tons per year of granular pig iron. This production method, unique for Russia, is based on the reduction firing of briquetted mixture (iron-ore concentrate (IOC) + coal + dolomite) at 1350 – 1450 °C in an annular hearth furnace heated by generator gas synthesized from coal. The production complex includes the following key facilities:
– IOC unloading section with raw material silos;
– open coal yard and coal preparation section;
– coal gasification section for generator gas production;
– section for preparing the mixture (IOC + coal + dolomite), briquetting, and drying the briquettes;
– ore–coal briquette metallization section;
– section for processing and separation of metallization products;
– closed-loop water supply section;
– gas cleaning section.
The main process units of the plant under construction include:
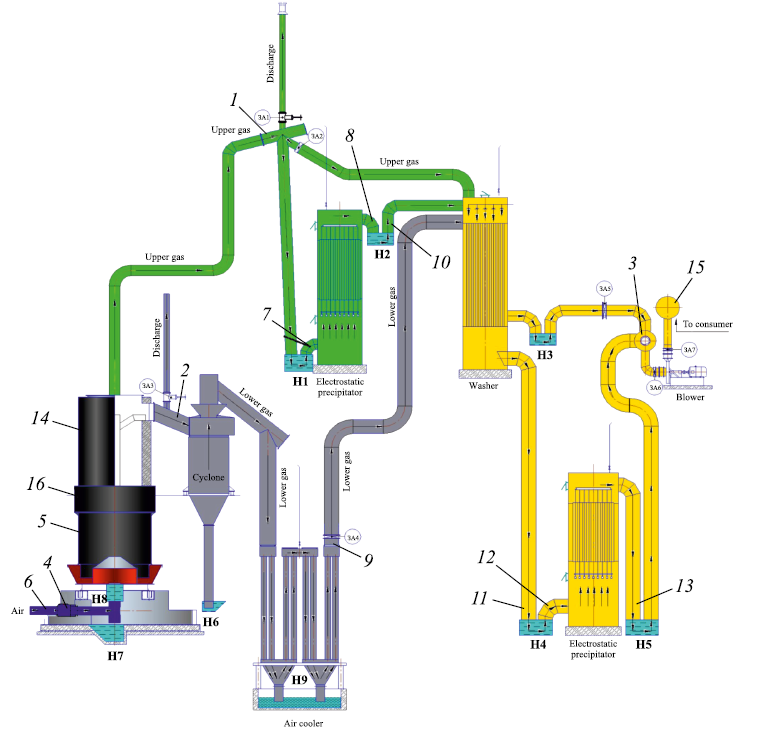
– two SM89 coal gasifiers manufactured in China, each with an inner diameter of 3.6 m, a cross-sectional area of 10.17 m2, and a height of 12.5 m, equipped with a generator gas purification system (Fig. 1);
Fig. 1. Gasifier with generator synthesis gas purification system: |
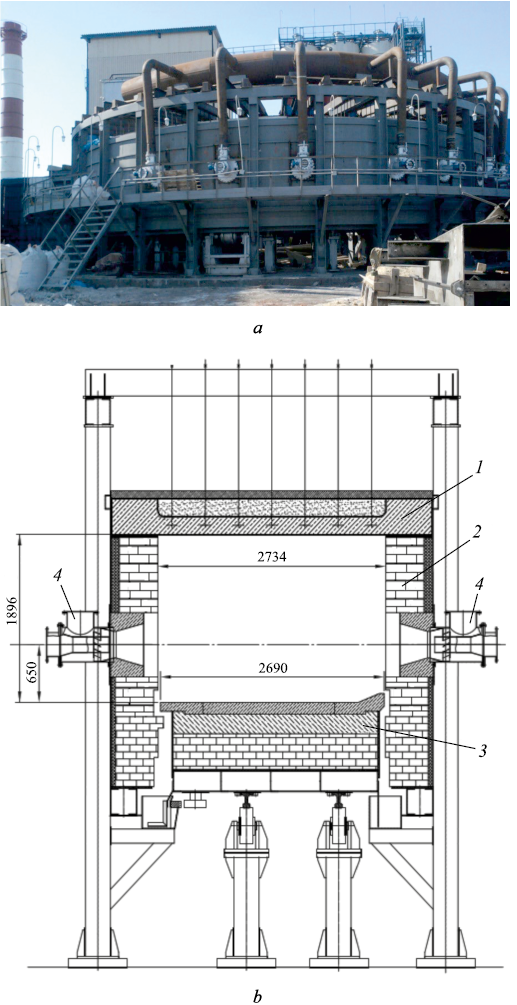
– annular furnace with a rotating hearth (Fig. 2). The outer diameter of the furnace is 20.99 m, the inner diameter is 15.6 m, and the hearth width is 2.69 m. Fourteen pairs of MS568 tuyeres are installed in the inner and outer walls of the furnace, positioned directly opposite each other so that the flame jets from opposing tuyeres meet and dissipate in the center of the furnace. The hearth rotation speed is variable: the time for one full revolution of the hearth can range from 27 to 45 min.
Fig. 2. Annular furnace for production of granular pig iron: |
Below is a brief description of the technological process for producing granular pig iron at the plant currently under construction. Raw materials are delivered to the ore yard by rail and truck. Coal and dolomite are transported by road, while iron-ore concentrate is delivered in open rail cars.
After crushing, the coal is screened on an inertial screen with 12 mm mesh openings. This separates it into two fractions: the oversize fraction, with particle sizes over 12 mm, is directed to the coal gasifier for the production of fuel synthesis gas. The undersize fraction, with particle sizes under 12 mm, is sent to a mill for further grinding to the particle size required for producing ore–coal briquettes. Briquettes with the composition of 80 % IOC + 20 % coal + 5 % dolomite are produced using a roller press. The maximum throughput of the press is 14 ± 0.5 tons per hour of ore–coal briquettes. To remove excess moisture and preheat the briquettes, they are fed into a dryer, where the drying agent is air heated in a recuperator to 350 – 400 °C using exhaust gases from the annular furnace.
Metallization of the iron-ore materials within ore–coal briquettes is carried out in an annular furnace with a rotating hearth. The furnace is divided into four technological sections:
– the coal bed loading section, where the “bed” serves as a protective layer for the refractory hearth lining and as a base for laying the briquettes;
– the briquette laying section, where briquettes are placed on the hearth;
– the reduction firing section, where the briquettes are metallized;
– the product discharge section, where the metallized product is removed from the hearth.
The first step involves loading the hearth with a coal “bed” using an apron feeder. The bed is evenly distributed over the hearth in a 3 – 5 cm thick layer. After the bed is in place, pre-dried and preheated ore–coal briquettes (heated to ~250 °C on a conveyor grate) are laid evenly on top of the bed in 1 – 2 layers.
As the hearth rotates, the briquette–bed “layer” is carried into the reduction firing section. The fuel synthesis gas, produced in the gasifier and preheated to 200 – 250 °C, is automatically distributed to the tuyers through a network of thermally insulated gas ducts, ensuring the required temperature conditions in the metallization zone. To improve combustion efficiency and reduce energy consumption, the air supplied to the tuyers is preheated to 400 – 450 °C in recuperators.
From the reduction firing zone, the metallized product enters the cooling and discharge zone, where it is removed from the hearth using water-cooled screw conveyors and fed through a water-cooled chute into a drag-type cooler. The cooled product is then transferred to a rotary screen, where the coal “bed” is first separated. The remaining agglomerated metallization product undergoes mechanical treatment, resulting in its breakdown into slag and granular pig iron. Next, the mechanical mixture of slag and pig iron is transported by conveyor to a magnetic separator, where the magnetic fraction is recovered – the target product of the process: direct reduced iron in the form of granules of pig iron.
Implementation of this innovative project began with the purchase and installation of the main and auxiliary equipment, and the construction of infrastructure. Alongside the installation of the annular furnace, coal gasifiers, and other systems, laboratory-scale studies were conducted on the metallization of ore–coal briquettes. The aim of these studies was to optimize briquette composition and refine the process of producing granular pig iron using the ITmk3 technology. The results of these studies are presented in this article.
Experimental studies and discussion of the results
One of the advantages of metallizing ore–coal materials in an annular furnace using ITmk3 technology is the formation of pig iron granules coated with a slag shell. This feature facilitates the separation of pig iron granules from the main slag mass [11 – 14] and reduces the cost of subsequent processing. An important requirement of ITmk3 technology is the use of iron ore concentrates with a total Fe content exceeding 60 %, as well as achieving a carbon content in the alloy within the range of 2.5 – 4.5 % after complete iron reduction. If the residual carbon content in the alloy is below 1.5 %, the melting point of the iron does not decrease significantly, and in this case, the furnace temperature must reach a maximum of around 1450 – 1550 °C. The guaranteed specifications for the final product (granular pig iron), according to the technology, are as follows (wt. %): >96 Fe; 2.0 – 4.0 С; 0.2 Si; 0.05 Р; 0.04 – 0.08 S [15 – 20]. However, the processes described in those publications are designed for high-calorific fuels, particularly natural gas.
The first stage of this study – aimed at determining the optimal conditions for metallizing IOC with coal – was carried out using thermogravimetry and the full factorial design method on a STA 449 Jupiter synchronous thermal analysis system. Samples were prepared according to the matrix of a full three-factor experimental design (Table 1), with the following control factors:
– Х1 – IOC-to-coal ratio (–1 → (70:30) % = 2,333; +1 → (80:20) % = 4).
– Х2 – coal particle size, μm (–1 → –50; +1 → –315).
– Х3 – lime additives, wt. % of the total (IOC + coal) –1 → 0; +1 → 5.
Table 1. Levels of control factors and variation intervals
| |||||||||||||||||||||||
Iron recovery into the alloy was used as the optimization parameter.
The experiments were conducted using sintering-grade iron ore concentrate from the Korshunovsky MPP with the following composition (wt. %, dry basis): 62.6 Fe2O3 ; 24.0 FeO; 3.95 SiO2 ; 2.654 Al2O3 ; 1.9 CaO; 4.0 MgO; 0.13 MnO; 0.255 TiO2 ; 0.04 SO2 ; 0.37 P2O5 ; 5.8 H2O; and 1.65 loss on ignition (LOI). Kasyanovsky coal from the Cheremkhovsky deposit was used as the reductant. Its composition (wt. %) was as follows: 77.3 total carbon (Ct ); 1.2 total sulfur (St); 16.5 ash (As); 11.5 moisture (as received); 13.7 total oxygen (Ot); 5.6 total hydrogen (Ht); 1.1 total nitrogen (Nt); and 45.6 volatile matter (Vt). The lower heating value of the coal was 23,028.5 kJ/kg. The ash fusion temperature ranged from 1310 to 1390 °C. The ash composition (wt. %) included: 67.1 SiO2 ; 19.2 Al2O3 ; 2.5 Fe2O; 2.2 CaO; 1.6 MgO; 0.7 K2О; 0.1 TiO2 ; 0.1 Na2O; 4.4 SO3 ; 0.01 MnO2 .
The prepared mixtures were pressed using a laboratory press at a pressure of 80 – 100 kg/cm2 to form briquettes measuring 8 mm in diameter and approximately 5 mm in height. The samples were heated from 40 to 1400 °C at a rate of 30 °C/min in an argon atmosphere. Sample masses ranged from 513 to 593 mg. Tests were carried out in a corundum crucible shaped as a shallow dish. During the experiments, the qualitative and quantitative composition of the gases released during thermolysis was monitored using an Aelos quadrupole mass spectrometer with an electron impact energy of 70 eV. The degree of iron metallization was calculated based on the composition of the released gases.
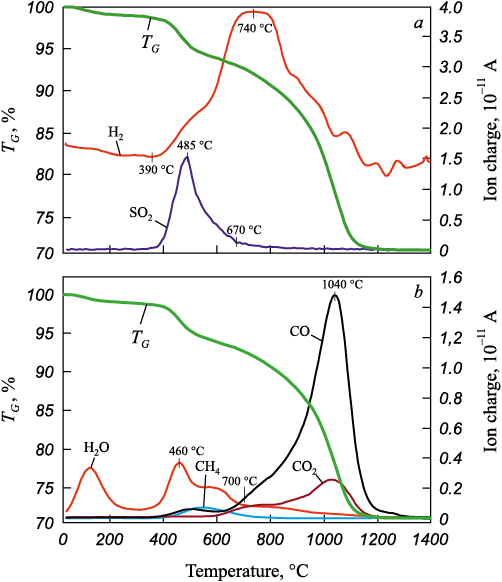
Eight experiments were conducted following a full three-factor experimental design. The sintering derivatograms of the iron ore concentrate, coal, and lime – along with the corresponding gas release profiles – were recorded in 24 figures (three per sample). Due to the overall similarity of the derivatograms, Fig. 3 presents the results of gas release analysis for representative Sample 6: Х1 = +1; Х2 = –1; Х3 = +1, which contained 80 % iron ore concentrate, 20 % coal, and 5 % lime (in excess of 100 %). The amounts of CO, CO2 , and H2O were determined from the derivatograms based on the area of the gas evolution effects, by comparison with those obtained for reference samples. The degree of IOC metallization was calculated based on the following reactions
| Fe3O4 + 4C → 3Fe + 4CO; | (1) |
| Fe3O4 + 2C → 3Fe + 2CO2 ; | (2) |
| Fe3O4 + 4H2 → 3Fe + 4H2O. | (3) |
Fig. 3. Derivatogram of IOC reduction by coal with composition |
The temperature range up to approximately 550 – 600 °С – corresponding to the first sharp mass loss – was excluded from the metallization calculations, as this process is associated with coal decomposition without interaction with the IOC. This was confirmed by the derivatogram of a separate sample of Cheremkhovsky coal (not shown in the article). Upon heating, the samples released CO, CO2 , H2O, and methane. The CO-to-CO2 ratio in the gas phase is indicative of the metamorphic grade of the coal. The calculations also accounted for the amount of water formed via reaction (3) within the 800 – 1000 °C temperature range.
Thermogravimetric analysis of the eight mixtures (IOC + coal + lime) revealed that gas release from the samples occurs in three distinct temperature intervals, °С: 40 – 210; 210 – 685; and 685 – 1400. In the first zone (40 – 210 °C), the release of hygroscopic moisture is observed, resulting in a mass loss ranging from 0.61 to 1.29 %.
In the second temperature interval (210 – 685 °C), the release of volatile compounds was observed. During this stage, sulfur was released predominantly in the form of SO2 , along with most of the CH4 , H2 , and H2O. The associated mass loss ranged from 5.46 to 8.6 %.
In the third zone (685 – 1400 °C), chemical interactions take place, leading predominantly to the formation of CO and CO2 . Mass loss in this range varies between 21.22 and 29.7 %. The mass of solid residue after firing the briquettes ranges from 60.64 to 72.48 %.
Table 2 presents the component and quantitative composition of the gas phase, calculated based on thermogravimetric data and used for computing the degree of iron metallization according to the experimental design matrix.
Table 2. Composition of the gas phase released during sintering of IOC
| ||||||||||||||||||||||||||||||||||||||||||||||||
Table 3 presents the results of iron metallization degree calculations based on reactions (1) – (3).
Table 3. Results of calculating the degree of samples metallization
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Based on the obtained data, the coefficients were calculated and a linear equation was derived to describe the dependence of iron recovery from IOC (Y1) on the IOC-to-coal ratio (Х1), coal particle size (Х2), and lime additives (Х3) expressed in coded coordinates.
| Y1 = 66.83 – 8.82Х1 – 4.16Х2 + 3.78Х3 + + 1.77Х1Х2 + 0.84Х1Х3 + 0.0037Х2Х3 + 1.29Х1Х2Х3 . | (4) |
Statistical evaluation of the coefficients in the derived equation showed that, at a 95 % confidence level, the coefficients for Х1 , Х2 , Х3 and the interaction term Х1Х2 , are statistically significant. As a result, Equation (4) is written in the reduced form as follows
| Y1 = 66.83 – 8.82Х1 – 4.16Х2 + 3.78Х3 + 1.77Х1Х2 . | (5) |
In actual (uncoded) values, Equation (5) takes the form
| \[\begin{array}{c}{Y_1} = 6.83 - 8.82\frac{{{X_1} - 3.167}}{{0.833}} - 4.16\frac{{{X_2} - 132.5}}{{82.5}} + \\ + 3.73\frac{{{X_3} - 2.5}}{{2.5}} + 1.77\frac{{{X_1} - 3.167}}{{0.833}}\frac{{{X_2} - 132.5}}{{82.5}},\end{array}\] | (6) |
where the numerators represent the zero values of the factor levels, and the denominators correspond to the variation intervals of the respective factors as listed in Table 1.
Based on the conducted thermogravimetric analysis of the samples, the following conclusions were drawn.
• The most significant factor affecting the degree of IOC metallization is the IOC-to-coal ratio; the next most influential factor is coal particle size.
• An increase in coal particle size (from –50 to –315 μm) raises the final temperature of the iron reduction process from 1255 to 1367 °C.
• The addition of lime intensifies the reduction process and shifts it to a lower temperature range (from 1367 to 1300 °C).
• The obtained results indicate that the metallization reaction proceeds in two stages, as evidenced by at least two sharp changes in the rate of mass loss (DTG), each corresponding to CO release. This fact can be interpreted as the direct reduction of iron oxides (the primary stage). The second stage involves the aggregation of molten iron droplets and the expulsion of gas from capillaries and inter-droplet spaces. The first stage begins at 640 °C with an increasing rate of mass loss, reaching up to 990 °C. This is followed by a stabilization and subsequent decrease in the rate of mass loss until about 1180 – 1250 °C. The second reduction stage then begins and completes within the temperature range of 1250 – 1360 °СС.
• The relatively low degree of iron metallization observed in this stage of the study (50 – 85 %) can be attributed to the following factors:
– the analysis and calculations were based solely on the gas phase;
– metallization occurred in an argon atmosphere;
– the Cheremkhovsky coal used in the experiments had a high degree of metamorphism;
– partial iron metallization may have occurred in the temperature range up to ~550 – 600 °C (corresponding to the first major mass-loss event), but this was not accounted for in the calculations;
• For effective reduction of iron oxides, it is advisable to use coal with a low volatile matter content and a high carbon content in briquette compositions.
To determine the operating conditions for the pig iron plant under construction, the next stage of the study was carried out, involving the preparation and firing of briquettes composed of iron ore concentrate, coal, and lime under laboratory conditions. A laboratory furnace with a portable hearth (Fig. 4) was designed for this purpose. The furnace is heated using gasified bituminous coal. Its internal dimensions are 500×500 mm in cross section and 650 mm in length. The furnace is equipped with a water-cooled flue gas duct and a forced gas extraction system. A platinum–platinum-rhodium thermocouple was installed to measure the furnace temperature. Before loading the briquettes, the furnace was preheated for 1.5 h to a temperature of approximately 1100 °С.
Fig. 4. Laboratory furnace with portable hearth on generator gas: |
Sintering-grade iron ore concentrate from the Korshunovsky MPP and Kasyanovsky bituminous coal from the Cheremkhovsky deposit were used to produce the briquettes. The furnace was heated using steam–air generator gas with the following composition (wt. %): 5.0 СО2 ; 0.2 О2 ; 27.0 СО; 13.0 Н2 ; 2.7 СН4 ; 0.3 С2Н4 ; 51.8 N2 . The lower heating value of the generator gas was 5976 kJ/m3.
The briquettes, pressed to a height and diameter of approximately 22 mm, were placed into ceramic pallets on a coke bed and fired on the portable hearth of the furnace (Fig. 5). The composition, mass before and after firing, and firing conditions are summarized in Table 4.
Fig. 5. Ore-coal briquettes during metallization in ceramic pallets
Table 4. Characteristics of IOC briquettes + coal + lime
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
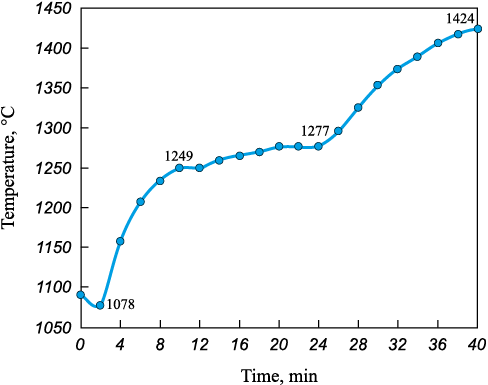
The briquettes were heated to a temperature of 1424 °C (Fig. 6). The initial temperature drop in the furnace to 1078 °C was due to the induction heating period after introducing the cold briquettes. In the second stage, the briquettes were intensively heated to 1249 °C at a rate of 21.4 °C/min over 8 min. The average gas temperature in the furnace during this stage was 1163 °С.
Fig. 6. Temperature-time graph of metallization of ore-coal briquettes |
In the third section of the temperature profile, within the 1249 – 1277 °C range, the heating rate decreased to approximately 2 °C/min. This segment effectively formed a temperature plateau, attributed to the endothermic effect of iron oxide reduction. The average gas temperature in the furnace during this stage was 1263 °С.
Further heating up to 1424 °C was required to establish the thermal conditions necessary for the liquid-phase separation of the metallic and slag phases [21]. The average heating rate over this 16 min interval was 9.2 °C/min. The average furnace gas temperature in this stage was 1350 °C. After drying and firing the briquettes for 40 min, the average yield of the final product was 69.3 ± 2.01 % (Table 4).
Fig. 7 shows the appearance of the briquettes after the completion of the metallization process. The inner part of the briquettes contains iron granules, while the outer surface is covered with an ash–slag shell, which partially protects the iron from oxidation.
Fig. 7. Ore-coal briquettes after metallization |
Briquette metallization proceeds according to the mechanism described in [22; 23]. The iron ore concentrate from the Korshunovsky MPP contains Fe2O3 and FeO oxides. Iron reduction from these oxides occurs in a stepwise manner, following the scheme: Fe2O3 → Fe3O4 → FeO → Fe.
Carbon monoxide acts as the primary reducing agent, synthesized through the interaction of coal carbon with carbon dioxide:
| С + СО2 = 2СО. | (7) |
A portion of the iron becomes carburized, forming iron carbide:
| Fe + С = Fe3С. | (8) |
When the carbon content in the reduced metal reaches 2 – 4 %, the melting point of the alloy decreases from 1539 °C to 1170 – 1380 °C. This reduction in melting temperature causes the carburized iron to transition into the liquid state. Due to cohesive forces, fine droplets of molten iron coalesce into larger ones, while the slag components of the charge remain solid. As the temperature of the charge increases to the ash fusion point (1310 – 1390 °C), the slag components also begin to melt. In the molten state, metal and slag do not mix due to their density difference and instead form separate phases. Upon cooling, the melt crystallizes to produce pig iron and slag.
As a result, the metallization process and formation of granulated pig iron proceed as follows: pelletized charge in the form of briquettes → reduction of iron oxides at 300 – 1200 °C → melting of iron at 1200 – 1300 °C with molten Fe3C emerging on the surface of the porous briquettes → slag melting at 1310 – 1390 °C and formation of distinct metal and slag phases → separation of metal and slag at ~1400 °C → cooling and solidification into pig iron and slag.
Table 5 presents the average composition of the metallization products from three batches of briquettes with the following formulation: 80 wt. % IOC + 20 wt. % coal + 5 wt. % lime (in excess of 100 wt. %), as characterized in Table 4.
Table 5. Composition of the products obtained as a result of metallization
| |||||||||||||||||||||||||||
It should be noted that the generator gas used – purified from tar compounds – exhibits low emissivity, approximately 0.2 – 0.3. At such low emissivity levels, radiative heat transfer during the heating of ore–coal briquettes is insufficiently effective. The radiative heat flux from the tyuer flame to the surface of the heated material can be significantly increased – by approximately 20 – 25 % – by enhancing the emissivity of the gas stream through flame carburization [24]. This can be accomplished by injecting a small amount of finely ground, low-ash dry coal or off-spec calcined petroleum coke into the flame’s reduction zone. As a result, the emissivity of the gas stream increases by a factor of 1.5 to 2.0, leading to higher flame luminosity, improved heat transfer to the briquettes, and a reduction in the time required to reach the target temperature.
Conclusions
Thermogravimetric analysis and laboratory-scale metallization experiments on ore–coal briquettes in a chamber furnace have demonstrated the feasibility of producing granulated pig iron on an industrial scale in a rotating hearth furnace using generator gas derived from coal gasification as the fuel.
The required metallization temperatures (1450 – 1550 °C) can be achieved by burning generator gas and recovering heat from exhaust gases to preheat the incoming air, fuel, and briquettes. If needed, heat transfer within the furnace can be further intensified by carburizing the flame using finely ground dry coal or calcined petroleum coke.
Key findings from the thermogravimetric studies are as follows:
• the IOC-to-coal ratio is the most influential factor affecting the degree of iron metallization.
• increasing the coal particle size raises the final temperature of the iron reduction process from 1255 to 1367 °C.
• the addition of lime intensifies the reduction process and shifts it to a lower temperature range (from 1367 to 1300 °C).
• to ensure effective reduction of iron oxides, the briquette composition should include coal with minimal volatile content and maximum fixed carbon content.
Based on the laboratory studies, a temperature–time mode was developed for the firing of ore–coal briquettes that ensures a high degree of iron metallization (80 – 87 %) when firing at temperatures between 1080 – 1424 °C for 40 min. The yield of metallized briquettes under these conditions was 66.45 %.
According to the design material balance, the annual production of granulated pig iron for the considered furnace setup will be 100,000 tons.
This coke-free method of producing granulated pig iron is particularly suitable for small-scale applications, especially where metallurgical and coal production facilities are located within the same regional economic area, allowing for simplified logistics and reduced transportation complexity.
References
1. Anikin A.E., Galevskii G.V., Rudneva V.V. Technological modes of efficient metallization of iron-oxide-containing waste from metallurgical production. Izvestiya. Ferrous Metallurgy. 2020;63(5):335–343. (In Russ.). https://doi.org/10.17073/0368-0797-2020-5-335-343
2. Yaroshenko Yu.G., Gordon Ya.M., Khodorovskaya I.Yu. Efficient and Resource-Saving Technologies of Modern Metallurgy: Manual. Yekaterinburg: LLC UIPC; 2012:670. (In Russ.).
3. Rashnikov V.F., Dubrovskii B.A., Galkin V.V. Method of metallization of iron-ore raw materials to produce granulated cast iron. Patent RF no. 2490332. MPK C21B 11/06, C21B 13/08. Bulleten’ izobretenii. 2013;(23). (In Russ.).
4. Grigor’ev V.G., Patkin P.G., Tepikin S.V. Furnace for obtaining reduced metal. Patent RF no. 92522. MPK F27B 3/06, C21B 13/10. Bulleten’ izobretenii. 2010;(8). (In Russ.).
5. Grigor’ev V.G., Patkin P.G., Tepikin S.V. Furnace for obtaining reduced metal. Patent RF no. 94680. MPK F27B 3/06, C21B 13/10. Bulleten’ izobretenii. 2010;(15). (In Russ.).
6. Grinberg I.S., Grinberg A.I. Annular furnace with rotary hearth. Patent RF no. 134532. MPK С21В 13/10, F27B 3/06. Bulleten’ izobretenii. 2013;(32). (In Russ.).
7. Chernykh V.E., Grigor’ev V.G., Tepikin S.V., etc. Technological line for production of metallized product. Patent RF no. 87166. MPK C22B 13/08, C21B 13/14. Bulleten’ izobretenii. 2009;(27). (In Russ.).
8. Grigor’ev V.G., Patkin P.G., Tepikin S.V., etc. Technological line for production of metallized product. Patent RF no. 93802. MPK C21B 13/14. Bulleten’ izobretenii. 2010;(13). (In Russ.).
9. Storozhev Yu.I., Podborskii L.N., Khudyakov I.A. Metallization of molded ore-coal materials in circular furnace. Izvestiya. Ferrous Metallurgy. 2015;58(4):235–240. (In Russ.). https://doi.org/10.17073/0368-0797-2015-4-235-240
10. Storozhev Yu.I., Podborskii L.N., Khudyakov I.A. Cooling of metallization products in annular furnace. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2015;1382(2):33–36. (In Russ.).
11. Gorbachev V.A., Evstyugin S.N., Kopot’ N.N., Rybkin V.S., Shavrin S.V. Selecting technology for direct iron production. Stal’. 2006;12(6):42–46. (In Russ.).
12. Yunes R., Opryshko I.A., Loboda P.I. Analysis of technologies of direct metal oxides reduction in circular furnaces. Vestnik NTUU “KPI”. 2011;61:184–192. (In Russ.).
13. Dubrovskii B.A., Shilyaev P.V., Redin E.V., etc. Metallization of
14. spatic iron ores of the Bakalsky deposit and production of granulated iron. In: Proceedings of the VI Int. Conf. “Energy Saving Technologies in Industry, Combustion Equipment, Environment Protection”. Moscow: NUST “MISIS”; 2012:178–182. (In Russ.).
15. Kikuchi S., Ito S., Kobayashi I., Tsuge O., Tokuda K. ITmk3 process. KOBELCO Technology Review. 2010;29:77–84.
16. Tanaka N., Miyagawa K., Harada T. FASTMET, FASTMELT, and ITmk3. Development of new coal-based ironmaking processes. Direct from Midrex, RHF Technologies. 2007/2008:8–13.
17. Kobayashi I., Tanigaki Y., Liagami A. A new process to produce iron directly from fine ore and coal. Iron and Steelmaker. 2001;28(9):19–22.
18. Sohn I., Fruehan R.J. The reduction of iron oxides by volatiles in a rotary hearth furnace process: Part I. The role and kinetics of volatile reduction. Metallurgical and Materials Transactions B. 2005;36:605–612. https://doi.org/10.1007/s11663-005-0051-y
19. Gordon Y., Els J. ITmk3 technology and its application for mining and steel industry in Ukraine and Russia. In: Int. Conf. on Ironmaking Technology (Kyiv, 21 March 2007). Kyiv; 2007:128–131.
20. Ishikawa H., Kopfle J., Mcclelland J., Ripke J. Rotary hearth furnace technologies for iron ore and recycling applications. Archives of Metallurgy and Materials. 2008;53(2):541–545.
21. Rutherford S.D., Kopfle J.T. Mesabi Nugget: World’s first commercial ITmk3 Plant. Iron and steel Technology. 2010;7:38–43.
22. Asanov A.V., Roshchin A.V., Roshchin V.E. Liquid-phase separation of products of solid-phase reduction of ferro-vanadium concentrates. Vestnik YuUrGU. Metallurgiya. 2010;(13(189)):37–40. (In Russ.).
23. Wegman E.F., Zherebin B.N., Pokhvisnev A.N., etc. Metallurgy of Cast Iron. Textbook for Universities. Moscow: Akademkniga; 2004:774. (In Russ.).
24. Patrushov A.E. Development of pyrometallurgical technology for extracting iron and zinc from electric steelmaking dust: Extended Abstract of Cand. Sci. Diss. Irkutsk; 2021:19. (In Russ.).
25. Gushchin S.N., Kazyaev M.D., Kryuchenkov Yu.V., etc. Theory and Practice of Heat Generation: Textbook. 2nd ed. Ekaterinburg. UGTU-UPI; 2005:379. (In Russ.).
About the Authors
B. P. KulikovRussian Federation
Boris P. Kulikov, Dr. Sci. (Chem.), Leading Researcher of the Chair “General Metallurgy”
79 Svobodnyi Ave., Krasnoyarsk 660041, Russian Federation
Yu. I. Storozhev
Russian Federation
Yurii I. Storozhev, Cand. Sci. (Eng.), Assist. Prof.
79 Svobodnyi Ave., Krasnoyarsk 660041, Russian Federation
A. S. Potapenko
Russian Federation
Aleksandr S. Potapenko, Cand. Sci. (Eng.), Assist. Prof. of the Chair of Technosphere and Environmental Safety
79 Svobodnyi Ave., Krasnoyarsk 660041, Russian Federation
Review
For citations:
Kulikov B.P., Storozhev Yu.I., Potapenko A.S. Metallization of ore-coal briquettes in an annular furnace heated by generator gas. Izvestiya. Ferrous Metallurgy. 2025;68(4):383-394. https://doi.org/10.17073/0368-0797-2025-4-383-394