Scroll to:
Assessment of the technological possibility of joint processing of ilmenite and perovskite concentrates
https://doi.org/10.17073/0368-0797-2025-5-444-453
Abstract
An urgent task facing the modern metallurgical industry is to increase the complexity of using mineral and technogenic raw materials by developing new technologies based on the principle of joint processing of raw materials from deposits that differ in the mineral composition of the ore component, for example, titanium-containing ores – ilmenite and perovskite. Joint processing of titanium-containing ores will improve the environmental and economic efficiency of processing domestic mineral raw materials, and will also create prerequisites for the development of titanium dioxide production in Russia. In order to scientifically substantiate the feasibility of joint processing of different types of titanium raw materials, the effect of temperature, reducing agent consumption and concentrate ratio on the phase formation process during carbothermic reduction of concentrate mixtures was established using thermodynamic modeling. The distribution of target metals by interaction products is considered, optimal parameters for the process of formation of rich titanium slags are proposed. The authors assessed the prospects for the associated extraction of rare and rare-earth metals. Thermodynamic analysis of the process of carbothermic reduction of mixtures, performed on model compositions of perovskite and ilmenite concentrates, showed that at low values of the perovskite concentrate / ilmenite concentrate (PC/IC) ratio, one can expect the formation of high-titanium slags with a TiO2 content of more than 80 %. However, concentration of Nb extracted into the alloy and content of rare earth elements in the slag will decrease several times compared to their initial values in the perovskite concentrate. At a PC/IC ratio of 1, it is possible to accumulate up to 2.5 % Nb in the alloy with a TiO2 content of up to 74 % in the slag. The advantage of joint processing of ilmenite and perovskite raw materials by the pyrometallurgical method is the ability to obtain rich titanium slags and selectively concentrate rare metals in the metallic phase, separating them from titanium, and rare earth metals in the slag within the framework of a single process flow sheet.
Keywords
For citations:
Agafonov S.N., Udoeva L.Yu., Vusikhis A.S., Leont’ev L.I. Assessment of the technological possibility of joint processing of ilmenite and perovskite concentrates. Izvestiya. Ferrous Metallurgy. 2025;68(5):444-453. https://doi.org/10.17073/0368-0797-2025-5-444-453
Introduction
Titanium is widely used in the paint and coatings, chemical, and other industries both as a metal and as a pigment-grade dioxide. The cessation of titanium raw material imports to the Russian Federation has resulted in a shortage of pigment titanium dioxide on the domestic market. At the same time, Russia possesses rich deposits of titanium ores [1] and their development is included in the near-term plans for the industry’s growth. The main industrial type of titanium deposits in Russia is represented by zircon–rutile–ilmenite placers, which account for up to 60 % of titanium production. The second most significant are primary deposits of ilmenite and ilmenite–titanomagnetite ores. A large portion of titanium reserves is concentrated in perovskite–titanomagnetite ores. Processing of these ores remains problematic: on the one hand, perovskite ores are polymetallic, and the presence of rare and rare-earth metals makes them promising; on the other hand, no efficient technology for the comprehensive processing of such raw materials currently exists.
At present, in the Kola Peninsula, in addition to the extraction of ilmenite ores (Gremyaha–Vyrmes deposit), work has begun on the development of the Afrikanda perovskite–titanomagnetite deposit, whose ores contain, in addition to titanium-bearing minerals, niobium and rare-earth elements (REEs) [2 – 5].
The efficiency of subsequent TiO₂ production steps largely depends on the method used for ilmenite concentrate decomposition. According to published data, a variety of preparation techniques have been developed for this purpose. These methods are generally divided into pyrometallurgical and hydrochemical processes, both aimed at separating iron oxides from titanium dioxide.
Carbothermic reduction of ilmenite concentrates to produce high-titanium slags (75 – 85 % TiO2 ) and hot metal is a well-established process. Numerous variations of this technology are typically related to the characteristics of the feedstock or to specific production objectives [6 – 8]. Smelting of the concentrate is performed in an electric arc furnace at temperatures up to 1600 °C, using carbonaceous reducing agents such as coke or anthracite. Flux-free operation yields slags with a residual FeO content of 10 – 12 %. The addition of lime or soda ash [9] increases the recovery of iron into the hot metal, lowering its residual concentration in the slag to 3 – 5 % FeO. In both cases, the process is conducted at 1600 – 1650 °C, and the titanium recovery to the slag remains nearly identical.
During beneficiation of Afrikanda ores, two main products are obtained – titanomagnetite and perovskite concentrates. The latter contains (wt. %): 48 – 50 TiO2 ; 33 – 35 CaO; 2 – 4 REEs; 0.9 – 1.2 (Nb, Ta)2O5 [10]. Technologies developed at the Kola Science Center of the Russian Academy of Sciences for processing perovskite concentrate rely on hydrochemical decomposition methods employing nitric acid, sulfuric acid, or mixtures of these acids with hydrochloric acid [11 – 14].
The pyrometallurgical processing of perovskite concentrates has been investigated far less extensively, since hydrometallurgical decomposition methods are currently considered more practical. Nevertheless, acid-based technologies present serious environmental challenges, generating large volumes of aggressive wastes – acidic effluents, sludges, and off-gases – that require costly neutralization. These drawbacks diminish the overall cost-effectiveness of processing such complex titanium feedstocks. In contrast, the application of carbothermic reduction to titanium-bearing materials (ilmenite concentrate, rutile, titanium slag, etc.) [15; 16], including perovskite concentrate [17], may offer new opportunities for pyrometallurgical routes in titanium production.
High-titanium slags obtained by reduction smelting of ilmenite concentrates are known to be highly refractory and “short” [18; 19]. Their viscosity depends primarily on the FeO and TiO2 contents and on the slag basicity (CaO/SiO2 ) [20; 21]. To improve fusibility, calcium oxide is added to adjust the CaO/TiO2 ratio toward the eutectic composition with a melting point of 1460 °C [22]. Building on existing ilmenite-processing technologies, the feasibility of using perovskite concentrate as a calcium-bearing fluxing additive has therefore been considered. Joint processing of ilmenite and perovskite concentrates offers clear advantages. Within a single integrated process flow sheet it enables the production of titanium-rich slags while simultaneously recovering rare and rare-earth metals.
To justify this approach, a thermodynamic analysis of the reactions between mixed concentrates and carbon was carried out to evaluate the feasibility of their joint carbothermic reduction.
Materials and methods
Samples of ilmenite concentrate (IC) and perovskite concentrate (PC) were used in this study. The chemical and phase compositions of the concentrates are presented in Table 1. X-ray diffraction, scanning electron microscopy, and energy-dispersive X-ray spectroscopy analyses revealed that titanium in the IC sample is concentrated in rutile (TiO2 ) and in the product of ilmenite leucoxenization – pseudorutile (Fe2O3·3TiO2 ). In the PC sample, the main ore minerals are perovskite (CaO·TiO2 ), titanite (CaTiSiO5 ), and ulvöspinel (Fe2TiO4 ). Rare metals, primarily niobium, are contained in loparite (5.0 %), ancylite (1.9 %), and thorite (26.5 %), while rare-earth elements (Ce, La, Nd) occur in perovskite (2.8 %) and loparite (22.8 % Ce).
Table 1. Chemical composition of ilmenite and perovskite concentrates
|
To optimize process parameters and calculate the equilibrium composition of products and the main technological indicators, thermodynamic modeling was performed using the HSC Chemistry 6.12 software package (Outotec Oy) [23]. The model inputs were based on compositions of working materials close to those of the concentrate samples used in subsequent experimental studies. Since the program database contains no information on pseudorutile, it was substituted by ilmenite and rutile according to reaction (1), as reduction of pseudorutile proceeds through the sequential formation of ilmenite and then dititanate:
| Fe2Ti3O9 + C = 2FeTiO3 + TiO2 + CO(g); | (1) |
| FeTiO3 + TiO2 = FeTi2O5 . | (2) |
For simplification, and considering that the process is mainly influenced by the ore mineral components, the thermodynamic analysis was carried out using model compositions of PC and IC with elemental ratios corresponding to real titanium raw materials (wt. %):
– perovskite concentrate: 60 CaO·TiO2 , 10 TiO2 , 10 SiO2 , 10 Fe2TiO4 , 9 CaCO3 , 1 Nb2O5 ;
– ilmenite concentrate: 40 FeO·TiO2 , 50 TiO2 , 8 Fe2O3 , 2 SiO2 .
Results and discussion
An important factor in evaluating the efficiency of joint processing of titanium raw materials is determining the optimal PC/IC ratio in the charge for carbothermic reduction roasting.
Equilibrium in the chemically reacting PC–IC–carbon systems was analyzed for PC/IC ratios of 0.2, 0.5, 0.8, and 1.0 as a function of temperature (700 – 1700 °C) and carbon consumption. Calculations were performed for 100 kg of mixture plus carbon in an inert atmosphere containing 100 mol N2 .
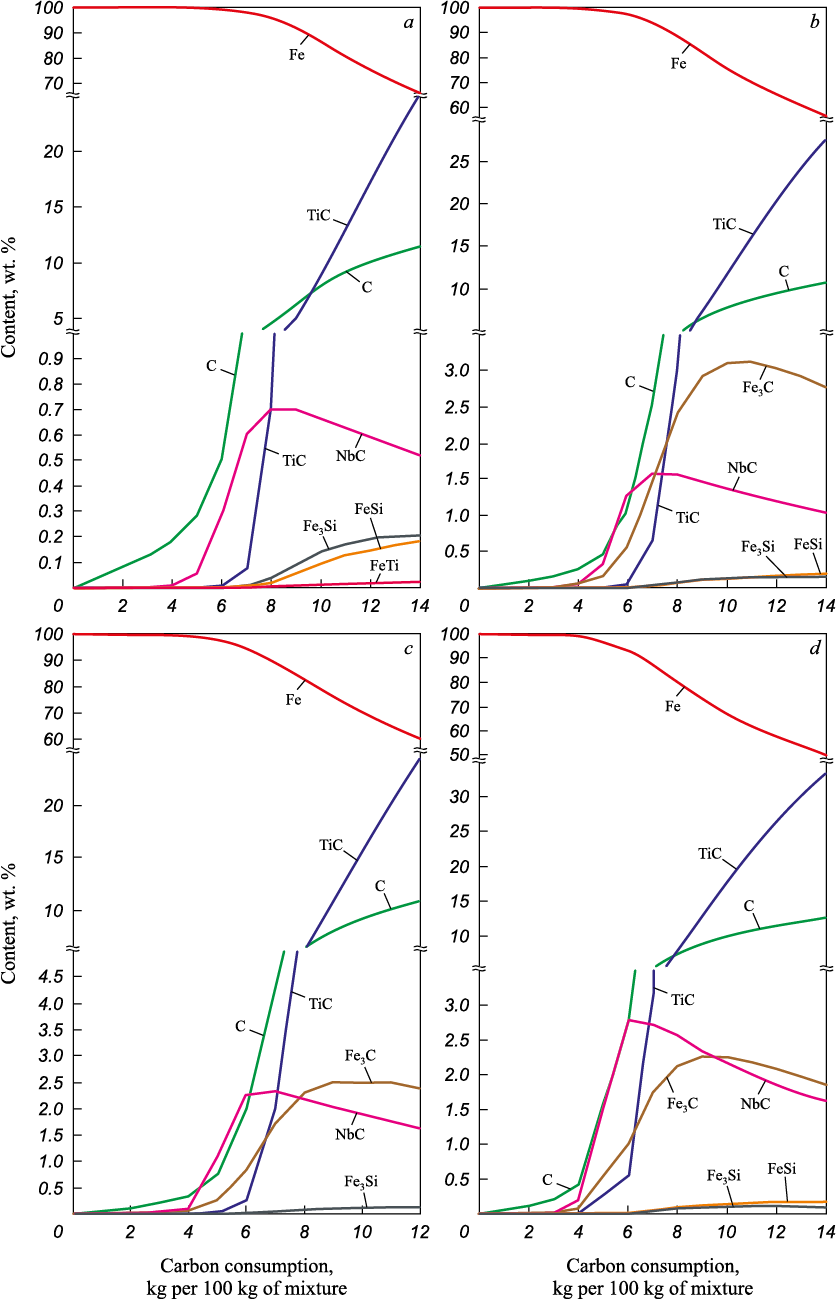
Regardless of the component ratio, the metallic product – in which iron forms the base of the alloy – contains niobium and titanium carbides and iron silicides, whose amounts increase in proportion to carbon consumption (Fig. 1). As the PC/IC ratio increases, the niobium content in the alloy (as NbC) rises from 0.7 to 2.7 %, reflecting its higher initial concentration in the mixture. At 1500 °C, noticeable transfer of niobium into the alloy occurs when carbon consumption exceeds 4 kg per 100 kg of mixture, irrespective of the PC/IC ratio. Titanium appears in the alloy only at higher carbon contents: above 6 kg C for PC/IC = 0.2 – 0.4, above 5 kg C for PC/IC = 0.8, and above 4 kg C for PC/IC = 1.0.
Fig. 1. Dependence of equilibrium composition of the slags from reduction |
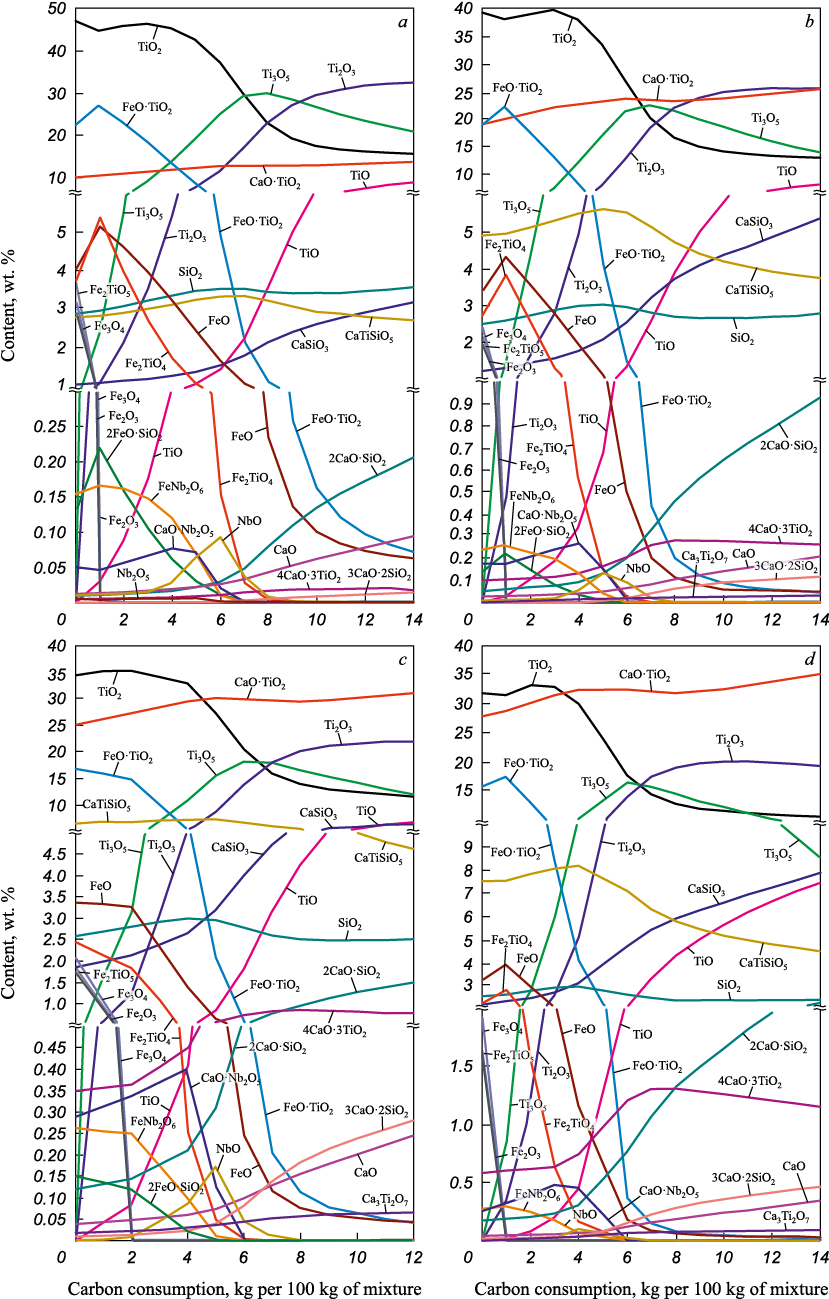
With an increasing PC fraction, the TiO2/CaO·TiO2 ratio in the slag also rises – from 4.5 at PC/IC = 0.2 to nearly equal proportions (1.1) at PC/IC = 1.0 (Fig. 2). According to the model, greater carbon consumption promotes the formation of lower titanium oxides, mainly through reduction of titanium from rutile (TiO2 ) and ilmenite (FeO·TiO2 ), i.e., the mineral phases of the ilmenite concentrate. The perovskite (CaO·TiO2 ) content changes only slightly, increasing smoothly from 5 to 8 % over the entire range of carbon additions. At 1500 °C, titanium reduction from rutile and ilmenite starts at low carbon levels and reaches a maximum (for Ti3O5 ) at 6 – 8 kg C per 100 kg of concentrate mixture. With increasing PC fraction, the proportion of lower oxides (Ti3O5 , Ti2O3 , TiO) decreases, improving the technological properties of the slag.
Fig. 2. Dependence of equilibrium composition of the alloys from reduction |
Table 2 presents the equilibrium compositions of reduction products of IR and PC mixtures at 1500 °C. For each PC/IC ratio, carbon consumptions corresponding to the onset and maximum of niobium transfer into the alloy were selected. Before niobium recovery, the metallic phase corresponds to low- or medium-carbon steel (<0.25 or ≤0.55 % C). Complete niobium transfer to the metallic phase as carbide yields cast iron (hot-metal).
Table 2. Equilibrium compositions of the interaction products of mixtures
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The effect of temperature on the equilibrium composition of carbothermic products was calculated for a PC/IC = 1.0 mixture at carbon consumption of 4 and 6 kg per 100 kg of charge, corresponding to the onset and maximum niobium recovery. Niobium appears in the alloy above 900 °C regardless of carbon level. In the temperature range above 1200 °C, where NbC formation is maximal, titanium also enters the alloy as carbide, reaching 0.5 % at 1500 °C. At higher temperatures, metallic niobium and iron silicides form.
The amount of titanium reduction products in the slag increases sharply with temperature. For Ti3O5 and Ti2O3 , their contents rise by two orders of magnitude – from fractions of a percent to 10 and 15 % at 4 and 6 kg C, respectively. Monoxide TiO forms above 1000 °C; its growth with temperature is more sensitive to carbon level.
Up to 1100 °C, iron reduction from ilmenite leads to an increase in rutile, whose content then drops rapidly as TiO2 is further reduced to lower titanium oxides.
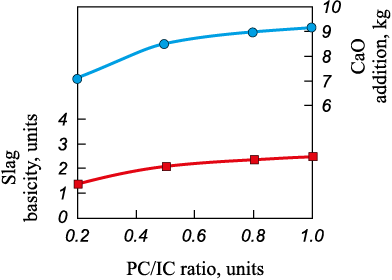
Throughout the temperature range, the concentration of tetravalent titanium compounds bound with calcium – perovskite (CaO·TiO2 ) and sphene (CaTiSiO5 ) – changes only slightly, confirming the stability of Ti(IV) within calcium-bearing minerals. Therefore, introducing CaO flux additives into the carbothermic smelting charge is advisable. Such additions inhibit the formation of lower titanium oxides, preventing the development of high-melting slags with elevated fusion/crystallization temperatures. According to [18; 25], when processing high-titanium raw materials, easily fusible slags with crystallization temperatures of 1400 – 1450 °C and a TiO2 content of approximately 60 % are obtained at a basicity (CaO/SiO2 ) of 4.0. In the present calculations, for mixtures with a PC/IC ration equal to 0.2 – 1.0, the modeled slag basicity increases from 1.4 to 2.5 (Fig. 3), governed primarily by the initial CaO/SiO2 ratio in the PC, since the IC contains ≤0.3 % CaO and ~2 % SiO2 . To condition the slag to the target CaO/SiO2 = 4.0, CaO flux must be added to the charge; for PC/IC = 1, about 9.2 kg CaO per 100 kg of mixture is required (Fig. 3).
Fig. 3. Calculated dependence of basicity of the slags |
Thus, thermodynamic analysis of carbothermic reduction of the model mixtures using model PC and IC compositions showed that at low PC/IC ratios, the formation of high-titanium slags (>80 % TiO2 ) can be expected. However, the niobium concentration in the alloy and the REE content in the slag decrease several-fold compared with their initial values in the perovskite concentrate. At a PC/IC ratio equal to 1, up to 2.5 % Nb can be accumulated in the alloy, with up to 74 % TiO2 in the slag (Table 2). In this case, the REE concentration in the slag decreases by no more than 1.5 times.
To predict the product composition from reduction smelting of a PC–IC mixture, thermodynamic modeling of the process was performed over 700 – 1700 °C. The calculations used the complete chemical and mineralogical compositions of the titanium concentrate samples (Table 1) at a PC/IC ratio equal 1.
Temperature dependences of the equilibrium compositions of the reaction products were calculated for two levels of carbon consumption: 4 kg C per 100 kg of concentrate mixture, which prevents titanium transfer to the alloy in the form of carbide, and 6 kg C, which ensures maximum niobium recovery to the alloy. Flux additions were set at 10 kg CaO per 100 kg of concentrate mixture. The charge compositions for both variants are presented in Table 3. According to the calculations, at a low carbon consumption (4 kg per 100 kg of mixture), the alloy mainly consists of iron with small amounts of chromium (0.6 %) and carbon (0.5 %) (Table 4). At 1500 °C, the NbC content in the alloy reaches only hundredths of a percent. Titanium (IV) in the slag is present mainly as CaO·TiO2 (up to 45 %), TiO2 (up to 22 %), and CaTiSiO5 (10 %), as well as magnesium titanates MgTiO3 and MgTi2O5 in amounts of 1.0 – 1.5 %. The remaining titanium occurs as suboxides: 2 % Ti2O3 , 4 % Ti3O5 , and 0.5 % TiO. The total titanium content in the slag, recalculated as TiO2 , is 59.2 %. Niobium from iron and calcium niobates is also reduced with increasing temperature, forming lower oxides NbO2 and NbO. Cerium from CeO2 is partially reduced to Ce3+AlO3 , while remaining completely in the slag.
Table 3. Composition of the charge of carbothermic reduction of mixtures
Table 4. Calculated composition of the interaction products of a mixture
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
At carbon consumptions higher than the stoichiometric requirement for complete iron reduction (6 kg C), the alloy contains 3 % Nb in the form of carbide and 2.0 % Cr, with the recovery of both elements into the metallic phase approaching 100 % (Table 4). Tantalum also completely transfers to the alloy as carbide (0.06 % TaC), similarly to niobium. Titanium reduction to carbide starts at 1000 °C and reaches its maximum at 1500 – 1600 °C, corresponding to 0.7 % Ti. With further increase in carbon content, its concentration rises to 4.4 wt. %, exceeding that of niobium. By carbon content, the metallic product corresponds to cast iron (4.3 % C). An increase in carbon consumption to 6 kg does not significantly change the qualitative composition of the slag. Quantitatively, with increasing temperature, the proportions of niobium suboxides NbO and NbO2 decrease sharply, amounting to less than 0.01 and 0.02 %, respectively, at 1500 °C, while the content of lower titanium oxides increases to about 10 %. The cerium concentration remains constant, both in the forms in which it occurs in the slag and in their relative proportions. It should be noted that for the selected mixture composition (PC/IC = 1), the cerium concentration in the slag (0.4 % CeO2 ) is about 1.5 times lower than in the original perovskite concentrate (0.6 % CeO2 ). Other components of the raw materials (Al, Mg, Si) originating from gangue minerals are present in the slag as simple or complex (binary and ternary) oxides, but their total content is small, limited to a few percent. According to the calculations, the introduced amount of flux (10 kg CaO) is insufficient to obtain slags with the desired basicity. At carbon consumptions of 4 and 6 kg, the CaO/SiO2 ratios are 3.4 and 2.7, respectively. Thus, in the first case, the charge should contain 15 kg CaO, and in the second – 20 kg CaO. If the calcium deficiency is compensated by increasing the PC proportion in the mixture, the resulting titanium slag should have a higher content of rare and rare-earth metals.
Conclusions
The advantage of joint pyrometallurgical processing of ilmenite and perovskite raw materials lies in the possibility of producing titanium-rich slags and selectively concentrating rare metals in the metallic phase, separating them from titanium, while rare-earth elements remain concentrated in the slag. Moreover, this approach eliminates the need to beneficiate perovskite–titanomagnetite ore to obtain a concentrate by removing iron-bearing minerals and calcite.
According to the thermodynamic models obtained, the joint reduction of ilmenite and perovskite concentrates proceeds with the formation of an iron-based alloy and a high-titanium slag containing more than 70 % TiO2 . The composition of the smelting products depends on both temperature and carbon consumption. At 1500 °C and carbon inputs below the stoichiometric requirement for niobium transfer to the alloy, the metallic phase corresponds to low- or medium-carbon steel (≤0.55 % C). When niobium is completely transferred to the metallic phase in the form of carbide, the product corresponds to hot metal.
At low PC/IC ratios (<0.5), high-titanium slags with a TiO2 content exceeding 80 % are expected to form. However, under these conditions, the niobium content in the alloy remains low, and the REE concentration in the slag decreases severalfold relative to their initial levels in the ore. Reduction of the concentrate mixture with a PC/IC ratio equal 1 at 1500 °C and 6 kg of carbon per 100 kg of mixture enables complete transfer of niobium to the alloy, which contains up to 3.0 % Nb, while producing a titanium-rich slag with 62 – 74 % TiO2 . In this case, the cerium concentration, as well as that of other REE, decreases by no more than 1.5 times.
References
1. Bykhovskii L.Z., Tigunov L.P. Titanium raw materials of Russia. Rossiiskii chimicheskii zhurnal. 2010;54(2):73–86. (In Russ.).
2. Nikolaev A.I., Gerasimova L.G., Petrov V.B., Mayorov V.G. Perovskite concentrate - as a source for titanium and rare-metal products. Kompleksnoe ispol'zovanie mineral'nogo syr'ya (KIMS). 2015;(2(293)):26–34. (In Russ.).
3. Krasotkin I.S., Voitekhovskii Yu.L., Leskov A.L. Afrikanda: beginning of the story. In: Proceedings of VIII All-Russian Fersman Scientific Session (April 18–19, 2011). 2011:23–30. (In Russ.).
4. Sokolov S.V., Nechelyustov G.N., Bystrov I.G. Alkaline magmatism of the Earth and related deposits of strategic metals. School “Alkaline magmatism of the Earth”. In: Proceedings of the XXXIII Int. Conf. Vernadsky Institute of Geochemistry and Analytical Chemistry of the Russian Academy of Sciences. 2016:116–118. (In Russ.).
5. Gerasimova L.G., Artemenkov A.G., Nikolaev A.I., Shchukina E.S. Titanium-rare metal concentrates from raw materials of the Kola region and the possibility of their joint processing to obtain scarce products. Arctic: Ecology and Economics. 2024;14(2):217–225. (In Russ.). https://doi.org/10.25283/2223-4594-2024-2-217-225
6. Reznicenko V.A., Ustinov V.S., Karyazin I.A., A.N. Petrun’ko A.N. Electrometallurgy and Chemistry of Titanium. Moscow: Nauka; 1982:276. (In Russ.).
7. Ma N., Warner N.A. Smelting reduction of ilmenite by carbon in molten pig iron. Canadian Metallurgical Quarterly. 1999;38(3):165–173.
8. Smirnov K.I., Gamov P.A. Pyro-metallurgical processing of ilmenite concentrate with production of iron and titanium oxides. Solid State Phenomena. 2021;316:385–389. https://doi.org/10.4028/www.scientific.net/ssp.316.385
9. Gudim Yu.A., Meirzhan A., Roshchin V.E. Possibilities of pyrometallurgical enrichment of ilmenite concentrates. Bulletin of the South Ural State University. Series: Metallurgy. 2016;16(1):23–32. (In Russ.). http://dx.doi.org/10.14529/met160103
10. Motov D.L. Technological solution to the perovskite problem. Proceedings of VII All-Russian Fersman Scientific Session (May 2–5, 2010). 2010:187–192 (In Russ.).
11. Goroshchenko Ya.G., Motov D.L., Belokoskov V.I., etc. On the issue and choice of a process flow sheet for obtaining titanium pigments from perovskite concentrate with concomitant extraction of rare metals. In: Proceedings of the Conf. on Chemical Technology of Mineral Resources of the Kola Peninsula. Leningrad: Academy of Sciences USSR; 1959;1:148–221. (In Russ.).
12. Kiselev Yu.G., Shchukina E.S. The solubility of the hydrated product obtained by perovskite nitric acid treatment in sulfuric acid. Bulletin of the Kola Science Center of the Russian Academy of Sciences. 2017;(2):81–86. (In Russ.).
13. Gerasimova L.G., Maslova M.V., Shchukina E.S. Preparation of titanium dioxide for heat-resistant sealants. Theoretical Foundations of Chemical Engineering. 2011;45(4): 511–516. https://doi.org/10.1134/S0040579513050035
14. Gerasimova L.G., Mel’nik N.A., Nikolaev A.I., Petrov V.B., Shchukina E.S., Bychenya Yu.G. Hydrochloric acid technology of perovskite concentrate and its radiation assessment. Ekologiya promyshlennogo proizvodstva. 2015;89:54–58. (In Russ.).
15. Rezan S.A., Zhang G., Ostrovski O. Carbothermal reduction and nitridation of ilmenite concentrate. ISIJ International. 2012;52(3):363–368.
16. Xiao J., Jiang B., Huang K., Jiao S., Zhu H-m. Selective reduction of TiO2–SiO2 in the carbothermal reduction of titanium raw materials for preparation og titanium oxycarbide. In: The Minerals, Metals &Materials Society: 7th Int. Symp. on High-Temperature Metallurgical Processing. 2016: 419–425. https://doi.org/10.1007/978-3-319-48093-0_52
17. Budin O.N., Kropachev A.N., Agafonov D.G., Cherepov V.V. Study into carbothermic method of titanium raw material decomposition in case of artificially synthesized perovskite. Izvestiya. Non-Ferrous Metallurgy. 2018;(5):23–30. (In Russ.). https://doi.org/10.17073/0021-3438-2018-5-23-30
18. Vasyutinskii N.A. Titanium Slags. Moscow: Metallurgiya; 1972:208. (In Russ.).
19. Asanov A.V., Anoshkin I.V., Mal’kov A.V., Senin A.V., Roshchin A.V. Influence of chemical composition and temperature on the viscosity of high-titanium slags. Bulletin of SUSU. 2008;(9):7–9. (In Russ.).
20. Jing J., Guo Y., Wang S., Chen F., Yang L., Yang J., Xu F., Yu L. Melting properties and phase composition transformation of Ti-bearing electric furnace slags in CaO–SiO2–MgO–Al2O3–50%TiO2. Metals and Materials International. 2024;30:2045–2056. https://doi.org/10.1007/s12540-024-01630-y
21. Yan Z.-m., Lv X.-w., Li Z.-s. Physicochemical properties and structure of titania-containing metallurgical slags: A review. Journal of Iron and Steel Research International. 2022;29:187–206. https://doi.org/10.1007/s42243-021-00678-z
22. Daněk V., Nerád I. Phase diagram and structure of melts of the system CaO–TiO2–SiO2 . Chemical Papers. 2002; 56(4):241–246.
23. Roine A. HSC Chemistry. Version 6.12 for Windows, Outotec Research Oy. Pori, Finland; 1974–2007.
24. Safonov A.V., Yakushevich N.F., Leboshkin V.N., Shadrin V.N., Gordin S.O. Carbothermic reduction of ilmenite concentrates in the solid phase. Izvestiya. Ferrous Metallurgy. 2004;47(2):19–22. (In Russ.).
25. Leont’ev L.I., Vatolin N.A., Shavrin S.V., Shumakov N.S. Pyrometallurgical Processing of Complex Ores. Moscow: Metallurgiya; 1997:432. (In Russ.).
About the Authors
S. N. AgafonovRussian Federation
Sergei N. Agafonov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. Yu. Udoeva
Russian Federation
Lyudmila Yu. Udoeva, Cand. Sci. (Eng.), Leading Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. S. Vusikhis
Russian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. I. Leont’ev
Russian Federation
Leopol’d I. Leont’ev, Academician, Adviser, Russian Academy of Sciences; Dr. Sci. (Eng.), Prof., National University of Science and Technology “MISIS”; Chief Researcher, Vatolin Institute of Metallurgy, Ural Branch of the Russian Academy of Sciences
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
32a Leninskii Ave., Moscow 119991, Russian Federation
Supplementary files
Review
For citations:
Agafonov S.N., Udoeva L.Yu., Vusikhis A.S., Leont’ev L.I. Assessment of the technological possibility of joint processing of ilmenite and perovskite concentrates. Izvestiya. Ferrous Metallurgy. 2025;68(5):444-453. https://doi.org/10.17073/0368-0797-2025-5-444-453