Scroll to:
Effect of boron oxide additives on viscosity and melting point of the CaO – SiO2 – Al2O3 – MgO system
https://doi.org/10.17073/0368-0797-2025-3-287-296
Abstract
The share of local iron ore raw materials of metallurgical enterprises of the Ural region is 50 – 60 %. The rest is brought from Central Russia, the Kola Peninsula and Kazakhstan. The issue of replacing imported raw materials with local, cheaper ones, is very relevant. The extraction of siderite iron ore of the Bakalskoye deposit (Southern Urals), the reserves of which are about 1 billion tons, is many times less than the mining and geological conditions allow because of the insignificant demand for this raw material due to its low quality. The high content of magnesium oxide in the ore makes blast furnace smelting difficult or impossible using more than 20 % of siderites in the charge. The basis of any blast furnace slag is a four-component system CaO – SiO2 – Al2O3 – MgO with the following composition, wt. %: 30 – 40 SiO2 , 31 – 49 CaO, 3 – 18 MgO, 7 – 20 Al2O3 . The melting point of such slags is 1280 – 1320 ℃. At a temperature of 1450 °C, their viscosity is about 0.5 Pa·s. An increase in the magnesium oxide content (>20 %) leads to a sharp increase in melting point of the slags, reduces the crystallization interval and makes them unstable. In this regard, the materials made from siderite ore using various technologies for preparing them for blast furnace smelting (raw ore, roasting-magnetic separation, agglomeration) are introduced into the blast furnace charge only as additives. Their share does not exceed 20 %. The effect of boric anhydride on the viscosity of high-magnesia blast furnace slags containing 15 – 36 % MgO was studied using modern methods of statistical processing of experimental data. It was shown that addition of boric anhydride to the initial charge allows to reduce the melting point of the slag and to increase the crystallization interval. This makes it possible to conduct blast furnace smelting on slags containing about 40 % MgO, which corresponds to a siderite share of 40 – 50 % in the initial charge.
Keywords
For citations:
Vusikhis A.S., Mikheenkov M.A., Leont’ev L.I., Agafonov S.N. Effect of boron oxide additives on viscosity and melting point of the CaO – SiO2 – Al2O3 – MgO system. Izvestiya. Ferrous Metallurgy. 2025;68(3):287-296. https://doi.org/10.17073/0368-0797-2025-3-287-296
Introduction
For blast furnace smelting to proceed efficiently, the slag formed during the process must possess stable physicochemical properties that are only slightly affected by fluctuations in chemical composition and temperature.
Slag melt viscosity is one of the most important physicochemical parameters that determine the stability and productivity of blast furnace operation. The final blast furnace slag should have good flowability during tapping. At tapping temperatures corresponding to those of steelmaking pig iron (1450 – 1550 °C), slag viscosity should be approximately 0.5 Pa∙s [1; 2].
In slags, the transition from solid to liquid occurs over a certain temperature range, so the melting point (Тm ) is a conditional value. The theoretical indicator of melting point is the liquidus temperature (Тl ) – the temperature at which the solid phase fully disappears upon heating. In practice, the melting point is considered the temperature at which slag begins to flow freely from the coke bed, which typically occurs when viscosity drops below 2.5 Pa∙s. This temperature is usually 200 – 300 °C lower than the tapping temperature and falls within the range of 1250 – 1350 ℃ [3].
Slag viscosity is greatly influenced by chemical composition, which is determined by the chemical and mineralogical makeup of the iron ore gangue, fluxes, and coke ash. It also depends on the nature of the smelting process, the thermal state of the furnace, and the grade of pig iron being produced. The content of major components in final slags at ironmaking plants across Russia, Ukraine, Europe, and the USA typically includes (wt. %: 30 – 40 SiO2 , 31 – 49 CaO, 3 – 18 MgO, 7 – 20 Al2O3 . Minor components include MnO (0.1 – 3.0 %), FeO (0.2 – 0.8 %), and S (0.8 – 2.2 %) [4; 5]. Excluding trace oxides (MnO, FeO, S), it is reliably established that the base of any blast furnace slag is the four-component system CaO – SiO2 – Al2O3 – MgO.
The relationship between slag viscosity and temperature in terms of these major components (CaO, SiO2 , Al2O3 , MgO) has been studied extensively, with findings presented in numerous works.
In melts containing less than 15 % alumina, an increase in basicity (R) from 0.6 to 1.5 and in MgO content from 0 to 20 % raises the melting point to 1350 – 1400 °C and narrows the solidification interval. The slags become “short” (i.e., less fluid). At temperatures below 1400 °C, slags with more than 25 % MgO exhibit poor flowability [6 – 9].
Changing MgO content from 0 to 25 % in slags with basicity within 0.6 – 1.5 leads to a decrease in viscosity to a minimum value that depends on alumina content and temperature. In acidic slags, viscosity decreases more significantly than in basic ones [10].
For slags containing 5 % aluminum oxide, the minimum viscosity of 0.15 Pa·s at 1500 °C is reached in the composition range of R ≈ 0.9 – 1.1, with 17 – 20 % MgO and 36 – 38 % SiO2 . Reducing the temperature to 1400 °C raises the minimum viscosity to 0.35 Pa∙s and expands the MgO range for achieving this minimum to 13 – 20 %, while shifting toward more acidic slags with 39 – 41 % SiO2 .
Raising the alumina content to 10 % increases the minimum viscosity. As the temperature decreases from 1500 to 1400 °C, viscosity rises from 0.2 to 0.3 Pa∙s. The composition range for achieving this minimum narrows from R ≈ 0.8 – 1.2, 13 – 24 % MgO, 35 – 40 % SiO2 (at 1500 °C) to R ≈ 1.05 – 1.2, 14 – 16 % MgO, and 39 – 41 % SiO2 (at 1400 °C).
With 15 % Al2O3 , the minimum viscosity increases further from 0.30 to 0.55 Pa∙s. The corresponding composition range narrows from R ≈ 0.9 – 1.2, 15 – 26 % MgO, and 30 – 33 % SiO2 to R ≈ 0.8 – 1.05, 18 – 22 % MgO, and 33 – 35 % SiO2 as the temperature drops from 1500 to 1400 °C. Increasing MgO content leads to a particularly sharp decrease in viscosity in acidic slags containing 25 – 35 % CaO. In slags with R ≈ 0.5 – 0.8, 13 – 18 % Al2O3 , and 16 – 25 % MgO, sufficient flowability is maintained at 1350 – 1400 ℃.
In slags containing 20 % Al2O3 , the melting point exceeds 1500 °C for any MgO content in the R ≈ 1.2 – 1.5 range. At R ≈ 1.1 – 1.2, crystallization occurs when MgO exceeds 16 %. As R decreases to 0.6, the critical MgO content increases to 20 %. When the MgO/Al2O3 ratio is approximately 0.5, Тl is close to 1450 °C at R ≈ 1.1 – 1.2 and decreases to 1350 °C when R drops to 0.6. In such slags, the minimum viscosity varies from 0.4 Pa∙s (at 1500 °C) to 1.0 Pa∙s (at 1400 °C) at SiO2 contents of 34 – 36 % [11 – 13].
Analysis of the data shows that in slags with basicity below 1.0, MgO content may reach 15 – 20 % without significantly complicating smelting, since the slags remain sufficient fluid and melt below 1350 °C. According to calculations [14], such slags form from blast furnace charges containing approximately 20 – 30 % siderite ore with 10 – 15 % MgO. A further increase in MgO content raises the slag melting point and leads to poor fluidity and process instability. Smelting with such charges becomes technically challenging or even unfeasible.
The gangue of siderite ores contains about 50 % MgO [15 – 17]. In this regard, in blast furnace smelting, siderite ore is used as an additive either directly in the initial charge or during sinter production. Smelting with a monocomponent charge of siderite ore from the Bakalskoye deposit is not feasible, since the resulting slags would have excessively high melting points. However, it is known [18 – 21] that the addition of boron oxide to blast furnace slags reduces their viscosity across the entire temperature range and makes them more fluid.
Методы и материалы исследования
Using a mathematical balance logical-statistical model, we assessed the feasibility of conducting blast furnace smelting with increased magnesium oxide content in the slag (from 15 to 30 %) due to the addition of 50 % roasting–magnetic separation concentrate (RMC). Within this framework, the effect of adding 1 – 3 % B2O3 on smelting parameters was evaluated [14].
According to the calculations, the changes in process performance parameters do not exceed 3 %. Productivity decreases, while coke consumption and total ore consumption increase. The addition of B2O3 reduces the content of all oxide components in the slag, including MgO, and introduces boron oxide into the system.
To evaluate the influence of boron oxide additions on the viscosity and melting point of the CaO – SiO – Al2O3 – MgO system, a simplex-lattice design of experiments was employed. This method provides a process model that closely reflects real-world behavior by accounting for the simultaneous influence of all varying factors.
Composition–property diagrams are geometric representations of multicomponent equilibrium systems. They include a compositional base, which reflects the chemical composition of the system, and a geometric property component (response surface) represented by a set of points, lines, or surfaces located above it. These diagrams provide quantitative information about specific properties corresponding to particular compositions within a multicomponent system.
However, constructing such diagrams requires a large number of experimental runs to determine how a property depends on the chemical composition of a multicomponent system.
The labor intensity of experimental studies necessitates optimizing the number of experiments. To achieve this, mathematical modeling is used in experimental design to analyze the influence of different components on the property under investigation and to obtain sufficiently complete information with minimal experimental effort. This approach significantly reduces the number of required experiments while ensuring that the results are obtained with an adequate level of reliability. One such method is the simplex-lattice approach, which analytically expresses the property–composition relationship as a continuous function [22].
In the simplex-lattice design of experiments, it is assumed that the properties of any mixture depend solely on the relative proportions of its components rather than on the total amount of the mixture. Examples of such properties include density, surface characteristics, viscosity, specific electrical conductivity of homogeneous metal and slag melts, gas solubility in solvent mixtures, and the hydrogen ion concentration (pH) of aqueous solutions, provided that their phase composition remains unchanged.
The preparation of the experimental design matrix was aimed at studying the methods of experimental planning, execution, and statistical processing of the results.
The matrix was developed based on the assumption that the target dependency could be approximated by a third-degree polynomial model, with the following initial conditions:
\(\frac{{{\rm{CaO}}}}{{{\rm{Si}}{{\rm{O}}_2}}}\) = R = 0.9 ÷ 1.2;
MgO = 15 – 36 %;
B2O3 = 0 – 15 %; Al2O3 = 5 – 20 %.
These starting conditions were based on the following: according to the calculations, increasing the share of siderite ore in the charge from 0 to 50 % results in SiO2 and MgO contents rising from 35 to 38 % and from 9 to 36 %, respectively, while CaO and Al2O3 contents decrease from 40 to 17 % and from 14 to 8 %, respectively. The addition of up to 3 % boric anhydride to the charge leads to up to 15 % B2O3 in the final slag.
Considering that MgO has significantly lower desulfurization capacity than CaO, the basicity of the slag was maintained in the range of 0.9 – 1.2. The MgO content in blast furnace slags was varied between 15 and 36 %, as concentrations below 15 % have little effect on the smelting process. In most final blast furnace slags produced during pig iron smelting in Russia and Western countries, the Al2O3 content varies from 5 to 20 %.
Thus, the studied composition field within the full five-component CaO – SiO2 – Al2O3 – MgO – B2O3 system is represented by a tetrahedron, the vertices of which correspond to pseudocomponents Y1 , Y2 , Y3 , and Y4. For each experimental slag composition in the design matrix, the precise content of each component was calculated (Table 1).
Table 1. Experiment matrix
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Synthetic slags were prepared for experimentation with CaO and SiO2 in a ratio corresponding to R = 0.9 – 1.2. The calcium oxide used (analytical grade) was pre-calcined in a muffle furnace at 910 °C for 6 h. The base samples were produced by heating and melting a mixture of oxides (CaO–SiO2 ) in a graphite crucible at 1500 – 1550 ℃ (30 min hold). The melt was poured into a mold and cooled.
The resulting slags were then blended in appropriate proportions with magnesium oxide (pre-calcined at 400 °C), aluminum oxide, and boric anhydride (pre-melted in a resistance furnace at 900 °C for 4 h). These mixtures were melted in a graphite crucible at 1500 – 1550 °C (30 min hold), poured into molds, cooled, and ground.
The powders were pressed into pellets, placed in a molybdenum crucible, heated to 1550 °C, and subjected to viscosity measurements. A vibrational viscometer operating in forced oscillation mode was used for this purpose [23; 24], with the melt temperature recorded using a tungsten–rhenium thermocouple. The measuring probe was made of molybdenum. Viscosity was measured during cooling at a rate of 5 – 7 °C/min.
Results and discussion
The results of the experiments are presented in Table 2 and Figs. 1 – 4.
Table 2. Experimental results
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
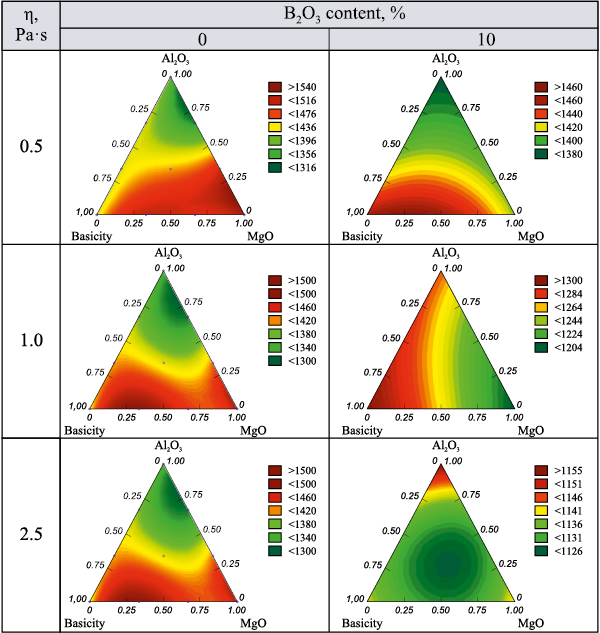
Fig. 1 shows the isolines of the response function for the temperature at which the given viscosity is achieved, with increasing B2O3 content from 0 to 10 %. According to the data in Fig. 1, an increase in B2O3 content in the slag leads to a decrease in the temperature required to achieve the specified viscosity, particularly in the simplex region with high MgO content.
Fig. 1. Isolines of the temperature response function at which a given viscosity |
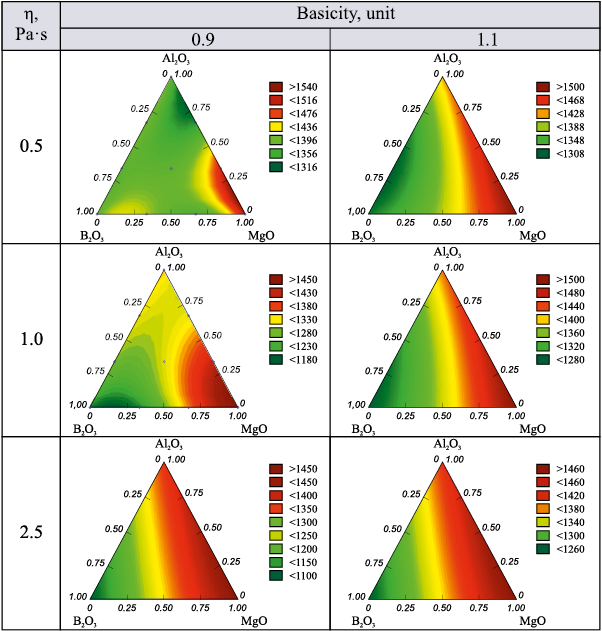
Fig. 2 presents the isolines of the response function for the temperature corresponding to the given viscosity, as the basicity of the slag increases from 0.9 to 1.1. As shown in the figure, higher basicity results in higher temperatures needed to reach the given viscosity in simplex regions with elevated MgO and Al2O3 content. At the same time, in regions with high B2O3 content, the required viscosity is achieved at relatively low temperatures – between 1100 and 1300 °C – regardless of the slag basicity.
Fig. 2. Isolines of the temperature response function at which a given viscosity |
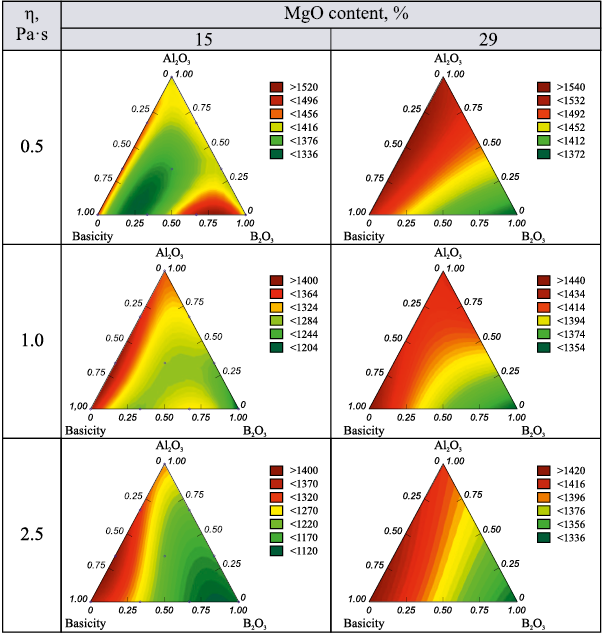
Fig. 3 shows isolines of the response function for the temperature at which the given viscosity is reached as MgO content increases from 15 to 29 %.
The results presented in Fig. 3 indicate that, despite nearly doubling the MgO content in the slag, in the simplex region with maximum B2O3 content, the increase in the temperature needed to reach the given viscosity is minor. The temperature remains below 1380 °C at minimum viscosity and below 1340 °C at maximum viscosity.
Fig. 3. Isolines of the temperature response function at which a given viscosity |
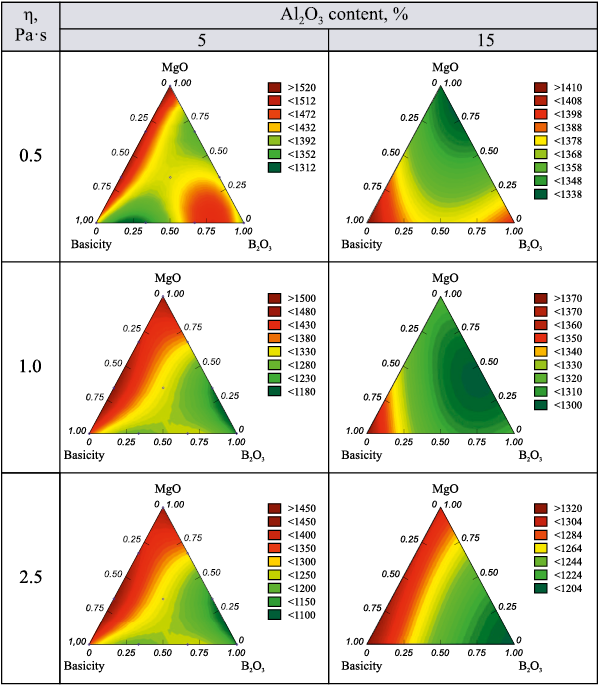
Fig. 4 presents isolines of the response function for the temperature at which the given viscosity is reached, as Al2O3 content increases from 5 to 15 %.
The results in Fig. 4 demonstrate that increasing the Al2O3 content in the slag lowers the temperature required to reach the desired viscosity in the region with high MgO content. This temperature is further reduced when B2O3 content is also increased.
Fig. 4. Isolines of the temperature response function at which a given viscosity |
Across the entire composition range of the CaO – SiO2 – Al2O3 – MgO –B2O3 system, in the absence of boron oxide, the temperature corresponding to the slag melting point (defined as the temperature at which viscosity reaches 2.5 Pa·s) exceeds 1390 °C. At basicity below 1.1, MgO content below 20 %, and Al2O3 content above 10 %, the melting point lies in the range of 1300 – 1400 °C. The viscosity of 0.5 Pa·s, which corresponds to the tapping viscosity, is reached at 1300 – 1440 °C for any basicity level, provided that MgO content is below 20 % and the Al2O3 /MgO ratio exceeds 0.5. Moreover, the higher this ratio, the lower the temperature required to reach that viscosity. This indicates that blast furnace smelting with slags of this composition can proceed without operational difficulties.
A further increase in magnesium oxide content causes a sharp rise in slag melting point – up to 1500 °C – alongside a narrowing of the crystallization interval. The tapping viscosity temperature rises to 1540 °C when MgO content reaches 36 %, making smelting under such conditions difficult or even infeasible.
The addition of B2O3 lowers the temperature at which the viscosity of the melt reaches 2.5 Pa·s, and at 15 % B2O3 , this temperature drops to below 1150 °C. the slags exhibit improved fluidity and greater thermal stability.
Conclusions
At present, the content of major components in most blast furnace slags is as follows (wt. %): 30 – 40 SiO2 , 31 – 49 CaO, 3 – 18 MgO, and 7 – 20 Al2O3 . Minor components include MnO (0.1 – 3.0 %), FeO (0.2 – 0.8 %), and S (0.8 – 2.2 %). Such slags exhibit good fluidity (viscosity below 0.5 Pa·s) at temperatures above 1450 °C. An increase in magnesium oxide content above 25 % significantly reduces slag fluidity and raises the melting point, making the slags viscous and difficult to process, which complicates blast furnace operation. For this reason, materials produced from siderite ore using various preparation technologies (raw ore, roasting–magnetic separation, agglomeration) are added to the blast furnace charge only as supplementary components. Their share in the initial charge is kept below 20 % to ensure that the resulting slag does not contain more than 15 – 20 % MgO.
The addition of boron oxide–containing materials to the initial charge lowers the slag melting point. This enables blast furnace smelting with slags containing approximately 40 % MgO, which corresponds to a siderite ore share of 40 – 50 % in the initial charge.
References
1. Efimenko G.G., Gimmel’farb A.A., Levchenko V.E. Metallurgy of Cast Iron. Moscow: Metallurgiya; 1988:308–323. (In Russ.).
2. Wegman E.F., Zherebin B.N., Pokhvisnev A.N., Yusfin Yu.S., Kurunov I.F., Paren’kov A.E., Chernousov P.I. Metallurgy of Cast Iron. Moscow: Akademkniga; 2004:774. (In Russ.).
3. Prikhod’ko E.V., Togobitskaya D.N. Physicochemical problems of melt formation in a blast furnace. In: Understanding Blast Furnace Smelting Processes. Bolshakov V.I., Tovarovskii I.G. eds. Dnipropetrovsk: Porogi, 2006; 2006: 248–276. (In Russ.).
4. Pliskanovskii S.T., Poltavets V.V. Equipment and Operation of Blast Furnaces. Dnepropetrovsk: Porogi; 2004:495. (In Russ.).
5. Badich A., Senk D., Gudenau H.W., Mavrommatis K.Th. Ironmaking. Aahen: RWTH Aahen University; 2008:402.
6. Bol’shakova L.I., Zhilo N.L. Physical properties of high-magnesia blast furnace slags during smelting of the Bakal siderites. In: Slag Mode of Blast Furnaces. Zhilo N.L., Ostroukhov M.Ya. eds. Moscow: Metallurgiya;1967:173–185. (In Russ.).
7. Zhilo N.L. Formation and Properties of Blast Furnace Slags. Moscow: Metallurgiya; 1974:120. (In Russ.).
8. Voskoboinikov V.G., Dunaev N.E., Mikhalevich A.G., etc. Properties of Liquid Blast Furnace Slags: Reference Book. Moscow: Metallurgiya; 1975:182. (In Russ.).
9. Saito N., Hori N., Nakashima K., Mori K. Viscosity of blast furnace type slags. Metallurgical and Materials Transactions B. 2003;34(5):509–516. https://doi.org/10.1007/s11663-003-0018-9
10. Kou M., Wu Sh., Ma X., Wang L., Chen M., Cai Q., Zhao B. Phase equilibrium studies of CaO–SiO2–MgO–Al2O3 system with binary basicity of 1.5 related to blast furnace slag. Metallurgical and Materials Transactions B. 2016;47(2): 1093–1102. https://doi.org/10.1007/s11663-016-0584-2
11. Liu Y., Lv X.W., Li B., Bai C.G. Relationship between structure and viscosity of CaO–SiO2–MgO–30.00 wt.% Al2O3 slag by molecular dynamics simulation with FT-IR and Raman spectroscopy. Ironmaking & Steelmaking. 2018;45(6): 492–501. https://doi.org/10.1080/03019233.2017.1288309
12. Shen F., Hu X., Zheng H., Jiang X., Gao Q., Han H., Long F. Proper MgO/Al2O3 ratio in blast-furnace slag: Analysis of proper MgO/Al2O3 ratio based on observed data. Metals. 2020;10(6):784. https://doi.org/10.3390/met10060784
13. Das K., Agrawal A., Reddy A.S., Ramma R.V. FactSage studies to identify the optimum slag regime for blast furnace operation. Transactions of the Indian Institute of Metals. 2021;74:419–428. https://doi.org/10.1007/s12666-020-02144-y
14. Vusikhis A.S., Leont’ev L.I., Agafonov S.N. Assessment of the efficiency of using Bakal siderites in blast furnace smelting. Izvestiya. Ferrous Metallurgy. 2022;65(7):504–510. (In Russ.). https://doi.org/10.17073/0368-0797-2022-7-504-510
15. Krasnoborov V.A., Yaroshevskii S.L., Denisov A.A., Rudin V.S., Biryuchev V.I., Polushkin M.F. Efficiency and Prospects of Using Siderite Ores in Blast Furnace Smelting. Donetsk; 1996:88. (In Russ.).
16. Yur’ev B.P., Melamud S.G., Spirin N.A., Shatsillo V.V. Technological and Thermal Engineering Bases of Siderite Ore Preparation for Metallurgical Processing: Monograph. Yekaterinburg: Den’ RA; 2016:428. (In Russ.).
17. Vusikhis A.S., Leont’ev L.I. Application of Siderite Ores in Iron and Steel Production: Monograph. Moscow; Vologda: Infra-Inzheneriya; 2022:116. (In Russ.).
18. Ren Sh., Zhang J., Wu L., Liu W., Bai Y., Xing X., Su B., Kong D. Influence of B2O3 on viscosity of high Ti-bearing blast furnace slag. ISIJ International. 2012;52(6):984–991. https://doi.org/10.2355/isijinternational.52.984
19. Kim G. H., Sohn I. Role of B2O3 on the viscosity and structure in the CaO–Al2O3–Na2O-based system. Metallurgical and Materials Transactions B. 2014;45(2):86–95. https://doi.org/10.1007/s11663-013-9953-2
20. Wang G., Wang J.-S., Xue Q.-G. Properties of boron-rich slag separated from boron-bearing iron concentrate. Journal of Central South University. 2018;25(4):783–794. https://doi.org/10.1007/s11771-018-3783-y
21. Vusikhis A.S., Leont’ev L.I., Gulyaeva R.I., Sergeeva S.V., Tyushnyakov S.N. Effect of B2O3 on viscosity of high-magnesia blast furnace slag. Izvestiya. Ferrous Metallurgy. 2023;66(1):89–96. https://doi.org/10.17073/0368-0797-2023-1-89-96
22. Kim V.A., Nikolai E.I., Akberdin A.A., Kulikov I.S. Planning an Experiment in the Study of the Physicochemical Properties of Metallurgical Slags: Methodological Manual. Alma-Ata: Nauka; 1989:116. (In Russ.).
23. Selivanov E., Gulyaeva R., Istomin S., Tyushnyakov S., Bykov A., Belyaev V. Viscosity and thermal properties of slag in the process of autogenous smelting of copper–zinc concentrates. Mineral Processing and Extractive Metallurgy. 2015;124(2):88–95. https://doi.org/10.1179/1743285514Y.0000000078
24. Vusikhis A.S., Selivanov E.N., Dmitriev A.N., Chentsov V.P., Ryabov V.V. Structure sensitive properties of system B2O3–CaO melts. Defect and Diffusion Forum. 2020;400:186–192. https://doi.org/10.4028/www.scientific.net/DDF.400.186
About the Authors
A. S. VusikhisRussian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
M. A. Mikheenkov
Russian Federation
Mikhail A. Mikheenkov, Dr. Sci. (Eng.), Leading Researcher of the Laboratory of Problems of Man-Made Formations
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. I. Leont’ev
Russian Federation
Leopol’d I. Leont’ev, Academician, Adviser, Russian Academy of Sciences; Dr. Sci. (Eng.), Prof., National University of Science and Technology “MISIS”; Chief Researcher, Institute of Metallurgy, Ural Branch of the Russian Academy of Science
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
32a Leninskii Ave., Moscow 119991, Russian Federation
S. N. Agafonov
Russian Federation
Sergei N. Agafonov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Rare Refractory Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Vusikhis A.S., Mikheenkov M.A., Leont’ev L.I., Agafonov S.N. Effect of boron oxide additives on viscosity and melting point of the CaO – SiO2 – Al2O3 – MgO system. Izvestiya. Ferrous Metallurgy. 2025;68(3):287-296. https://doi.org/10.17073/0368-0797-2025-3-287-296