Scroll to:
Thermodynamic analysis of conditions for iron and titanium separation in ilmenite concentrate by selective reduction of elements
https://doi.org/10.17073/0368-0797-2025-2-171-178
Abstract
The thermodynamic modeling method was used to determine the temperature of beginning of reduction of iron, vanadium, silicon, and titanium in ilmenite concentrate by carbon or hydrogen at different amounts of reducing agent in the system. The amount of excess carbon relative to the stoichiometry of the iron reduction reaction does not affect the temperature of reduction beginning, but determines their reduction degree and the amount of carbides formed. The amount of hydrogen in the system significantly affects the temperature of reduction beginning: with an increase in water amount, this temperature of each element decreases, but to a different extent. The wider temperature range of beginning of reduction by hydrogen and the quantitatively unequal effect of temperature create more opportunities for controlling the solid-phase selective reduction by hydrogen in comparison with carbon. In contrast to the carbothermic process, the solid-phase reduction of titanium by hydrogen is negligible at relatively low temperatures, at which titanium is reduced by carbon and forms carbides. The low solubility of hydrogen in solid iron excludes its influence on the behavior of elements at the stage of separation melting of solid-phase reduction products. This makes it possible to carry out reduction in hydrogen flow by changing the temperature and amount of hydrogen in the reducing gas mixture, and to control the processes of selective solid-phase reduction of elements. The use of hydrogen at the stage of solid-phase reduction makes it possible to selectively reduce iron with the storage of titanium oxides in the oxide phase in form of TiO2 , and after separation of the reduction products by melting, to obtain the products in demand (carbon-free iron and TiO2 concentrate).
Keywords
For citations:
Smirnov K.I., Gamov P.A., Roshchin V.E., Samolin V.S. Thermodynamic analysis of conditions for iron and titanium separation in ilmenite concentrate by selective reduction of elements. Izvestiya. Ferrous Metallurgy. 2025;68(2):171-178. https://doi.org/10.17073/0368-0797-2025-2-171-178
Introduction
Russia faces a shortage of raw materials for the production of pigment-grade titanium dioxide (TiO2 ) and metallic titanium. In 2019, the import of pigment-grade titanium dioxide amounted to 53.6 thousand tons, covering 67.5 % of domestic consumption [1]. Pigment-grade TiO2 is produced at the “Crimean Titan” plant (Armiansk) using the sulfuric acid process, while metallic titanium is manufactured using chloride technologies at the “VSMPO-AVISMA” facility (Berezniki) and the “Solikamsk Magnesium Plant” (Solikamsk).
Globally, the chloride process is the predominant method for producing pigment-grade titanium dioxide, as it has a lower environmental impact compared to the sulfuric acid process [2]. However, due to the lack of an efficient strategy for processing chlorination by-products, the chloride process typically relies not on ilmenite concentrate, but on natural rutile concentrates or high-titanium slags obtained through the pyrometallurgical Sorel process. The Sorel process, which involves electric arc smelting, is the primary pyrometallurgical method used to treat ilmenite concentrate. This process yields vanadium-rich pig iron and high-titanium slag. During smelting, 96 – 97 % of iron and 45 – 48 % of vanadium contained in the concentrate are transferred to the metallic phase. Titanium is partially reduced to lower oxides and, in some cases, to its metallic form, with up to 2 % of the titanium content entering the metal phase [3]. The Sorel process is highly energy-intensive due to the formation of a high-melting-point slag and is therefore employed primarily in regions with low-cost electricity [4].
A reduction in electricity consumption during the processing of ilmenite-based feedstock can be achieved by carrying out the reduction reactions in a separate unit designed for solid-phase metallization, using low-cost reductants and fuels. In this approach, the electric arc furnace is used solely for separating the metallization products. An assessment of using a metallized charge in electric arc smelting has shown that, at a metallization degree of approximately 70 %, the electricity consumption per ton of high-titanium slag is reduced by about 35 %, and by a further 20 % when the contribution of sensible heat is considered [5].
Based on pilot tests for processing concentrates from the Chineiskoe deposit, a two-stage pyrometallurgical process was proposed for the recovery of iron, titanium, and vanadium [6]. This technology involves the preliminary reduction of iron with carbon in a rotary kiln, achieving a metallization degree of 90 – 93 %, followed by separation of hot metallized concentrate in an electric arc furnace to obtain vanadium pig iron and titanium slag. Although this process has been recommended for other medium- and high-titanium concentrates, it has not yet been implemented on an industrial scale.
Hydrogen can serve as an alternative reducing agent for iron during the processing of titanium-bearing iron ore materials. Both theoretical studies [7; 8] and experimental research [9 – 11] have demonstrated that hydrogen offers several advantages over carbon-based reductants, which is particularly important for the selective reduction of metals from complex ores.
Thermodynamic modeling is widely used to assess the conditions under which chemical reactions occur in metallurgical processes. In most cases, thermodynamic calculations are based on evaluating chemical reactions through changes in Gibbs free energy. This approach involves identifying the most probable reaction from among the possible ones by comparing ∆G(Т) values. However, in complex systems such as ores and concentrates, this type of calculation is labor-intensive and, in some cases, unfeasible due to the large number of components involved. Therefore, the use of computer-based software packages is considered the most efficient approach for modeling complex systems.
The thermodynamic modeling of element reduction from ilmenite concentrate has been explored in numerous studies [12 – 21]. Reference [12] examined the distribution of titanium, iron, and impurities between the metal and slag phases during carbon reduction, depending on the temperature range (1550 – 1750 °C) and slag basicity. In [13], the influence of CO – H2 gas mixture composition was analyzed over a temperature range of 500 – 1200 °C. Study [14] investigated carbothermic reduction under vacuum conditions. According to [15], the lower titanium oxides formed during hydrogen reduction at temperatures above 827 °C can be used to reduce iron from ilmenite. Reference [16] examined the reduction process at 0.10132 MPa with varying amounts of ilmenite, carbon, and hydrogen. In [17], changes in Gibbs free energy were calculated for a number of reactions involved in the carbothermic reduction of elements in ilmenite across a broad temperature range (25 – 1650 °C). Study [18] also focused on carbothermic reduction from ilmenite concentrate under vacuum. In [19], the reduction process was studied using palm kernel biomass as a reductant at 1000 – 1200 °C. Reference [20] analyzed the influence of Na2CO3 on reduction during electric smelting. Study [21] explored reactions occurring in Ar – H2 gas mixtures with varying hydrogen content in 800 – 1000 °C temperature range. However, a comparative analysis of reduction conditions for elements in ilmenite concentrate using carbon versus hydrogen is lacking in the literature.
The objective of the present study is to compare, through thermodynamic modeling, the conditions of element reduction in ilmenite concentrate using carbon or hydrogen as the reducing agent.
Research methodology
Thermodynamic calculations were carried out using an ilmenite concentrate with the following composition (wt. %: O 42.6; Mg 0.4; Al 0.3; Si 0.7; Ti 24.0; V 0.3; Mn 0.4; Fe 31.3.
The equilibrium phase composition of the system components and the temperature sequence of their transformations during carbon or hydrogen reduction were determined using thermodynamic modeling with the Terra software package [22]. The calculations were based on the Terra thermodynamic database, supplemented by thermodynamic data for individual substances from reference sources [23 – 25]. The amount of reducing agent was set either at the stoichiometric level required for iron reduction or in excess. For carbon, the excess levels were 10, 20, 30, and 100 wt. %; for hydrogen, the amounts exceeded the stoichiometric requirement by factors of 10, 100, and 1000. The total pressure in the system was assumed to be constant at 0.10132 MPa. Equilibrium phase compositions were calculated over the temperature range of 750 – 1700 °C in 50 °C increments for carbon reduction and 500 – 1700 °C for hydrogen reduction. The calculated data on phase composition were tabulated and conventionally divided into three phases: metal (iron, titanium, vanadium, and silicon, including carbides and silicides), slag (oxides), and gas. The degree of metallization, as well as the composition of the metallic, slag, and gas phases, were analyzed and presented in the form of graphical dependencies for clarity.
Modeling results and discussion
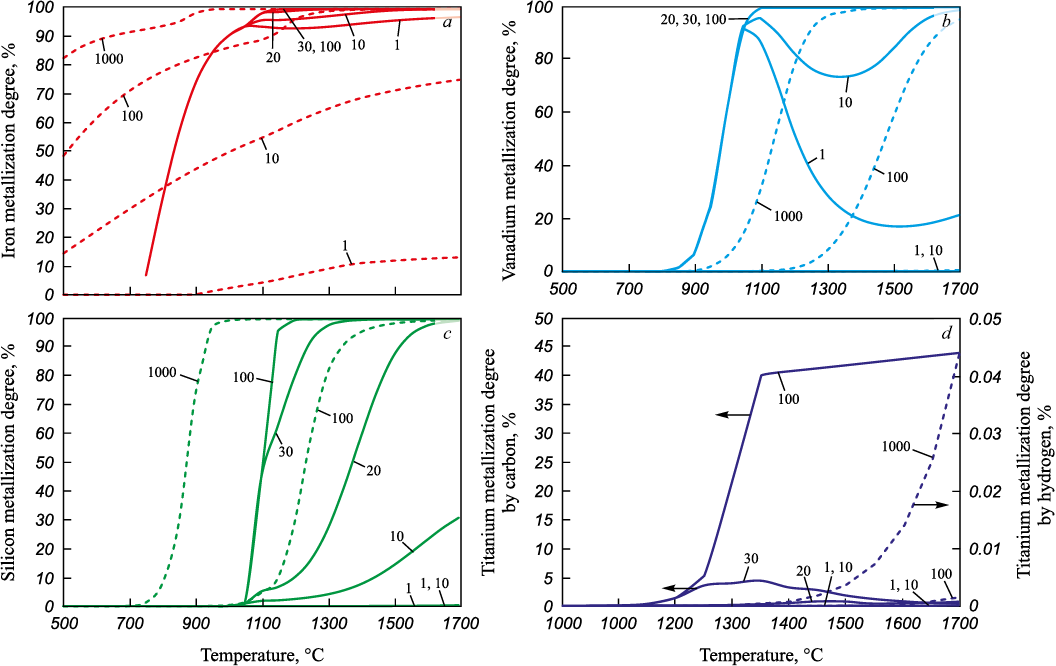
Based on the results of thermodynamic modeling, several elements in ilmenite concentrate – namely iron, titanium, vanadium, and silicon – were found to actively participate in the reduction process and contribute to the formation of the metallic phase (see Figure).
Effect of the amount of reducing agents on the degree of metallization of iron (a), |
Among these, iron is the most readily reducible element. Its reduction by both carbon and hydrogen occurs across the entire range of temperatures and reductant quantities examined, with the only exception being hydrogen at its stoichiometric amount. Below 1050 °C, the degree of iron metallization with carbon increases steadily, regardless of the amount of reductant present. Above 1050 °C, metallization continues to rise, but the rate of increase varies depending on the level of carbon excess.
An increase in the amount of hydrogen in the system promotes a higher degree of iron metallization. The maximum degree of iron metallization by carbon reaches 99.39 % in the temperature range of 1250 – 1700 °C at a 100 wt. % excess of reductant relative to the stoichiometric amount. When reduced by hydrogen, the maximum degree of metallization reaches 99.36 % at 1700 °C with a 1000-fold excess of hydrogen. In both cases, complete reduction is not achieved due to the formation of the complex oxide FeAl2O4 . In the carbon reduction system, iron in the metallic phase is primarily present in the form of iron carbide Fe3C, whereas in the hydrogen system it is found as a separate component. Reduced silicon binds part of the iron in the form of silicides FeSi and Fe3Si, forming in the carbon system at temperatures above 1050 °C, and in the hydrogen system at temperatures above 750 and 1200 ℃.
Vanadium reduction by carbon is observed across the entire studied temperature range, whereas reduction by hydrogen occurs between 750 and 1700 °C. In the 800 – 1050 °C range, carbon reduction results in a progressive increase in the equilibrium degree of vanadium metallization, with vanadium carbide (VC) forming in the metallic phase. In calculations using stoichiometric or slightly excessive (10 wt. %) carbon, the degree of vanadium metallization decreases above 1050 °C, followed by an increase at temperatures above 1500 °C for stoichiometric carbon and above 1350 °C for 10 wt. % excess. This behaviour is attributed to the formation of not only vanadium carbides but also silicon carbides. With carbon excesses of 20, 30, and 100 wt. % over stoichiometry, the vanadium metallization degree increases with temperature, reaching 100 % at 1100 ℃. In the case of hydrogen reduction, increasing the amount of hydrogen in the system leads to a decrease in the temperature at which vanadium begins to appear in the metallic phase. For stoichiometric hydrogen and 10- and 100-fold excesses, these threshold temperatures are 1450, 1200, and 950 °C, respectively. The maximum equilibrium degree of vanadium metallization is observed at 1650 – 1700 °C and reaches 99.99 %.
Silicon reduction by carbon occurs over the temperature range of 1000 – 1700 °C. At 1000 and 1050 °C, the degree of silicon metallization is independent of the amount of carbon. Above 1050 °C, the metallization degree increases with both temperature and carbon content. The maximum degree of silicon metallization is observed at 1700 °C and amounts to 30.83, 99.52, 99.9, 99.95, and 100.00 % for stoichiometric carbon and 10, 20, 30, and 100 wt. % excesses, respectively.
Silicon reduction by hydrogen at a 1000-fold excess relative to the stoichiometric amount is observed within the temperature range of 650 – 1700 °C. As the hydrogen content in the system increases, the temperature at which silicon enters the metallic phase decreases – down to 1250 and 900 °C for 10-fold and 100-fold excesses, respectively. At stoichiometric hydrogen levels, no silicon reduction is observed. Within the range of 1350 – 1700 °C, a maximum silicon metallization degree of 100 % is reached at a 1000-fold hydrogen excess. In both cases, silicon in the metallic phase is predominantly present as iron silicides FeSi and Fe3Si.
Titanium reduction by carbon occurs at temperatures of 1050 °C and above, while reduction by hydrogen is only observed at temperatures above 1550 °C and only in the case of a 1000-fold excess of hydrogen. When the carbon excess is below 10 wt. % of the stoichiometric amount, the titanium metallization degree remains negligible, reaching only 0.02 % at 1100 °C. With carbon excesses of 20, 30, and 100 wt. %, the maximum equilibrium degree of titanium metallization reaches 0.77, 4.37, and 44.02 % at 1450, 1350, and 1700 °C, respectively.
Solid-phase titanium reduction by hydrogen is not possible at stoichiometric levels or at hydrogen excesses below a factor of 100. The maximum degree of titanium metallization reaches only 0.05 % at 1700 °C, even at a 1000-fold excess of hydrogen over the stoichiometric amount. In the carbon system, titanium is present in the metallic phase in the form of titanium carbide (TiC), whereas in the hydrogen system it appears dissolved in the iron phase.
Selective reduction of iron from ilmenite is achievable with both carbon and hydrogen. For effective separation of the elements present in ilmenite concentrate, it is essential to achieve a high degree of solid-phase iron metallization while avoiding titanium metallization and ensuring that titanium is not reduced during the separation smelting of the solid-phase reduction products. Under the conditions required for high solid-phase iron metallization, other components of the ilmenite concentrate (vanadium, silicon, titanium) may also be partially reduced. However, titanium reduction is highly undesirable in this context. Vanadium, being one of the valuable components of ilmenite concentrate, can be recovered from both the metal and slag phases during further processing. However, the current technologies for vanadium recovery from the metal phase are associated with significant losses at all processing stages, which reduces the overall vanadium yield from the ore. Silicon, present in the concentrate as part of the gangue material, has no practical value when reduced to the metallic phase due to its low content.
The results of the calculations showed that iron reduction in ilmenite occurs at significantly lower temperatures when hydrogen is used as a reductant compared to carbon. Moreover, the degree of metallization is highly sensitive to the amount of hydrogen in the system. At the stoichiometric amount of hydrogen, the onset temperature of iron reduction is approximately 900 °C, while a 10-, 100-, or 1000-fold excess lowers this temperature to below 500 °C. In contrast, the onset temperature of iron reduction by carbon remains nearly constant at around 700 °C, regardless of the carbon excess.
Titanium is virtually not reduced by hydrogen, even when the hydrogen content exceeds the stoichiometric requirement for iron reduction by a factor of 1000. In contrast, in the presence of carbon, titanium forms a stable compound – titanium carbide (TiC) – which enables a relatively high degree of metallization. However, when carbon is limited, it is preferentially consumed in the reduction of other elements.
Vanadium exhibits similar behavior to titanium in the presence of carbon. However, due to its lower oxygen affinity, vanadium is reduced at comparatively lower temperatures – closer to those required for iron reduction. The extent of vanadium reduction by hydrogen depends strongly on the hydrogen content. At stoichiometric levels and even at a tenfold excess, vanadium is scarcely reduced. In contrast, at 100- and 1000-fold hydrogen excesses, the degree of metallization approaches 100 % at 1700 and 1400 °C, respectively. Notably, vanadium reduction by hydrogen occurs at higher temperatures than with carbon.
Unlike titanium and vanadium, silicon forms iron silicides. Its reduction is strongly influenced by the reductant’s chemical activity. In the presence of excess carbon, silicon reduction begins at 1000 °C. By contrast, hydrogen reduction depends on the partial pressure of oxygen in the system. Under high hydrogen excess – 100 and 1000 times the stoichiometric amount required for iron reduction – silicon appears in the metallic phase at 950 and 750 °C, respectively.
It is worth noting that the temperature at which elements enter the metallic phase during carbon reduction does not depend on the amount of carbon in the system. Instead, the carbon content determines the extent to which the reduction reactions proceed. In contrast, the onset temperatures of element reduction by hydrogen Тonset are strongly influenced by the H2O/H2 ratio in the system. For the system under study, the equilibrium values of the H2O/H2 ratio are presented in the Table.
Temperatures of the elements appearance in metallic phase and ratio
|
Conclusions
The temperatures at which iron, vanadium, silicon, and titanium appear in the metallic phase during reduction of ilmenite concentrate by carbon are 700, 800, 1000, and 1150 °C, respectively, and are not affected by the amount of carbon present in the system. The excess carbon content relative to the stoichiometric requirement for the iron reduction reaction determines the extent of vanadium, silicon, and titanium reduction as the system temperature exceeds the onset temperature for the reduction of the corresponding element. By adjusting the carbon content and the process temperature, it is possible to control the development of reduction reactions and achieve solid-phase selective reduction of iron or simultaneous reduction of iron, silicon, and vanadium while retaining titanium in the oxide phase.
The presence of carbon in excess of the stoichiometric amount for iron reduction leads to the formation of iron, vanadium, and silicon carbides. As the temperature increases during the pyrometallurgical separation stage, carbon contained in these carbides can partially reduce and bind titanium into carbides, thereby reducing the effectiveness of iron–titanium separation.
The onset temperatures for the reduction of iron, vanadium, silicon, and titanium from ilmenite concentrate by hydrogen span a broader range and are significantly influenced by the hydrogen content in the system. As the hydrogen concentration increases, the onset temperature for each element decreases, though to a different extent. This wider temperature window and the non-uniform temperature dependence of element reduction provide greater flexibility for controlling solid-phase selective reduction by hydrogen compared to carbon.
The negligible solubility of hydrogen in solid iron ensures that it does not affect the behavior of elements during the separation smelting of solid-phase reduction products. As a result, selective solid-phase reduction of elements can be carried out in a hydrogen stream, with process temperature serving as a control parameter for reduction selectivity. This approach eliminates the risk of titanium reduction and prevents the loss of titanium dioxide TiO2 during the separation of reduction products.
References
1. Leont’ev L.I., Volkov A.I. State and development of mineral resource base and metallurgical products to ensure Russia’s import independence. In: The Int. Sci. Conf. “Physico-Chemical Foundations of Metallurgical Processes” named after Academician A.M. Samarin. Vyksa. October 10-14, 2022. Vyksa; 2022:18–36. (In Russ.).
2. Bogatyreva E.V. Production of Refractory Rare Metals: Metallurgy of Titanium and Its Compounds. Moscow: NUST MISIS; 2019:161. (In Russ.).
3. Denisov S.I. Electrothermy of Titanium Slags. Moscow: Metallurgiya; 1979:165. (In Russ).
4. Alekseev L.F., Chentsov A.V., Shavrin S.V. Metallurgical evaluation of Ural ilmenite concentrates. In: Complex Processing of Metallurgical Raw Materials. Preprint. Yekaterinburg: UB RAS; 1994:27–35. (In Russ.).
5. Reznichenko V.A., Ustinov V.S., Karyazin I.A., Petrun’ko A.N. Electrometallurgy and Chemistry of Titanium. Moscow: Nauka; 1982:278. (In Russ.).
6. Reznichenko V.A., Solov’ev V.I., Burmistrova T.M. Metallurgical evaluation of titanium-magnetite concentrate of the Chineyskoye deposit. Kompleksnoe ispol’zovanie mineral’nogo syr’ya. 1986;(2):60–63. (In Russ.).
7. Roshchin V.E., Gamov P.A., Roshchin A.V., Salikhov S.P. Prospects for the development of hydrogen technologies in the domestic metallurgy. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2023;79(2):144–153. (In Russ.). https://doi.org/10.32339/0135-5910-2023-2-144-153
8. Roshchin V.E., Smirnov K.I., Gamov P.A., Roshchin A.V. Fundamental features of hydrogen reduction of metals and feasibility of using hydrogen at the present stage. In: Innovations and Complex Processing of Mineral Raw Materials – Actual Components of the Diversification of the Economy. Materials of the Int. Sci. and Pract. Conf. Almaty; 2023: 153–155. (In Russ.).
9. Gamov P.A., Smirnov K.I., Roshchin V.E., Vyatkin G.P. Hydrogen technologies for decarbonization of ferrous metallurgy: Scientific background and technical capabilities. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2024;80(7):47–53. (In Russ.). https://doi.org/10.32339/0135-5910-2024-7-47-53
10. Kosdauletov N.Y., Roshchin A.V., Roshchin V.E. Production of high manganese slag by reducing iron and phosphorus from ferromanganese ores with hydrogen. Chernye metally. 2024;(2):4–9. (In Russ.). https://doi.org/10.17580/chm.2024.02.01
11. Smirnov K.I., Gamov P.A., Samolin V.S., Roshchin V.E. Selective reduction of iron from ilmenite concentrate. Chernye metally. 2024;(7):19‒23. (In Russ.). https://doi.org/10.17580/chm.2024.07.03
12. Moosavi-Khoonsari E., Siahboumi A.A., Kwon S.Y., Jones R., Mostaghel S. Thermodynamic modeling of ilmenite smelting and impurity distribution. JOM. 2024;76: 6511–6533. https://doi.org/10.1007/s11837-024-06844-4
13. Xiao W., Lu X.-G., Zou X.-L., Li C.-H., Ding W.-Z. Multiple gaseous reduction of ilmenite: Thermodynamic and experimental study. Rare Metals. 2015;34:888–894. https://doi.org/10.1007/s12598-014-0264-9
14. Run H., Pengsheng L., Yuehui Y., Jinzhu Z. Vacuum carbothermic reduction of Panzhihua ilmenite concentrate: A thermodynamic study. Mineral Processing and Extractive Metallurgy Review. 2017;38(3):193–198. https://doi.org/10.1080/08827508.2017.1281129
15. Yu H., Li C., Wei K., Li Y., Ma W. Effect of titanium suboxides on the reaction mechanism of hydrogen-reduced ilmenite. International Journal of Hydrogen Energy. 2023; 48(5):1747‒1757. https://doi.org/10.1016/j.ijhydene.2022.10.071
16. Yunos N.F.M., Idris M.A., Nasrun N.A., Kurniawan A., Nomura T., Rezan S.A. Structural characterizations and phase transition on the reducibility of ilmenite ore with different carbon reductants by carbothermal reduction under hydrogen atmosphere. Journal of Sustainable Metallurgy. 2023;9:1716–1731. https://doi.org/10.1007/s40831-023-00760-8
17. He C., Zheng C., Dai W., Fujita T., Zhao J., Ma S., Li X., Wei Y., Yang J., Wei Z. Purification and phase evolution mechanism of titanium oxycarbide (TiCxOy) produced by the thermal reduction of ilmenite. Minerals. 2021;11(2):104. https://doi.org/10.3390/min11020104
18. Zhang G., Gou H., Wu K., Chou K. Carbothermic reduction of Panzhihua ilmenite in vacuum. Vacuum. 2017;143:199‒208. https://doi.org/10.1016/j.vacuum.2017.06.016
19. Setiawan A., Rhamdhani M.A., Pownceby M.I., Webster N.A.S., Harjanto S. Kinetics and mechanisms of carbothermic reduction of weathered ilmenite using palm kernel shell biomass. Journal of Sustainable Metallurgy. 2021;7(4): 1819–1837. https://doi.org/10.1007/s40831-021-00457-w
20. Myrzakulov M.K., Jumankulova S.K., Barmenshinova M.B., Martyushev N.V., Skeeba V.Y., Kondratiev V.V., Karlina A.I. Thermodynamic and technological studies of the electric smelting of Satpaevsk ilmenite concentrates. Metals. 2024;14(11):1211. https://doi.org/10.3390/met14111211
21. Wang Y., Yuan Z., Matsuura H., Tsukihashi F. Reduction extraction kinetics of titania and iron from an ilmenite by H2–Ar gas mixtures. ISIJ international. 2009;49(2):164‒170. https://doi.org/10.2355/isijinternational.49.164
22. Belov G.V., Trusov B.G. Thermodynamic Modeling of Chemically Reacting Systems. Moscow: Bauman MSTU; 2013:96. (In Russ.).
23. Thermodynamic Properties of Individual Substances. Guide. In 2 vols. Gurvich L.V., Khachkuruzov G.A., Medvedev V.A., etc. eds. Moscow: AN SSSR; 1962. (In Russ.).
24. Thermodynamic Properties of Individual Substances. Gurvich L.V., Veits I.V., Medvedev V.A., etc. eds. Moscow: Nauka; 1978–1982:623. (In Russ.).
25. JANAF Thermochemical Tables. NSRDS – NBS37. Washington: Gove Printing Office; 1971:1144.
About the Authors
K. I. SmirnovRussian Federation
Konstantin I. Smirnov, Research Associate of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
P. A. Gamov
Russian Federation
Pavel A. Gamov, Cand. Sci. (Eng.), Assist. Prof., Head of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
V. E. Roshchin
Russian Federation
Vasilii E. Roshchin, Dr. Sci. (Eng.), Prof., Chief Researcher of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
V. S. Samolin
Russian Federation
Vladislav S. Samolin, Postgraduate of the Chair “Pyrometallurgical and Foundry Technologies”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
Review
For citations:
Smirnov K.I., Gamov P.A., Roshchin V.E., Samolin V.S. Thermodynamic analysis of conditions for iron and titanium separation in ilmenite concentrate by selective reduction of elements. Izvestiya. Ferrous Metallurgy. 2025;68(2):171-178. https://doi.org/10.17073/0368-0797-2025-2-171-178
JATS XML