Scroll to:
Thermodynamic modelling of reduction of iron ore materials by hydrogen-containing gases
https://doi.org/10.17073/0368-0797-2025-2-163-170
Abstract
The article presents the results of studying the processes of reduction of iron ore titanomagnetite pellets with synthesis gas by means of thermodynamic modeling using the Terra software package. Its use made it possible to model and predict chemical and phase transformations in iron ore titanomagnetite pellets during reduction using hydrogen-containing synthesis gas, taking into account the effect of temperature, hydrogen concentration and other parameters on reduction. Calculations were performed with different gas mixture contents to evaluate the model efficiency. Content of the CO – N2 – H2 – CH4 gas mixture for calculations varied with an increase in CO and H2 , decrease in N2 and constant CH4 . Thermodynamic modeling showed that when balance of the main phases in high-temperature systems is achieved during reduction with various gas mixtures, the concentration of distribution of silicon, aluminum, titanium, magnesium, and calcium elements remains constant. Significant changes are observed in the concentration of iron, vanadium, and manganese, which is associated with the features of reduction process and composition of the gases used. Dependences of the system equilibrium composition on temperature at various element contents were obtained. The constructed thermodynamic model describes the reduction process and can be used to optimize it under various production conditions.
For citations:
Dmitriev A.N., Burova Yu.E., Vit’kina G.Yu., Barbin N.M., Terent’yev D.I. Thermodynamic modelling of reduction of iron ore materials by hydrogen-containing gases. Izvestiya. Ferrous Metallurgy. 2025;68(2):163-170. https://doi.org/10.17073/0368-0797-2025-2-163-170
Introduction
Currently, considerable attention is being paid to the development of various approaches to decarbonizing metallurgical production. The conventional blast furnace process for metal production involves the emission of large volumes of carbon dioxide into the atmosphere. One of the possible solutions is to move toward decarbonization without radical changes to the production process by capturing CO2 emissions followed by their utilization or storage. A more fundamental approach involves replacing carbon monoxide with pure hydrogen or, more feasibly, using synthesis gas – a combination of hydrogen and carbon monoxide – which can substitute a significant portion of solid carbon-based fuel in the blast furnace process and offers the potential for progress toward decarbonization while meeting high environmental standards [1 – 3].
One approach to reducing CO2 emissions during pig iron production is the injection of coke oven gas and blast furnace gas into the blast furnace in order to decrease the specific coke consumption. For the effective injection of blast furnace gas, the CO2 and H2O content must be minimized as much as possible [4 – 5]. In this regard, coke oven gas is far more suitable from a process standpoint – its CO2 content is approximately 3 vol. %. For instance, ArcelorMittal has announced the implementation of a coke oven gas injection technology at its plant in Spain1. The companies Dillinger and Saarstahl have invested €14 million in a new coke oven gas conversion plant for blast furnace injection at the Rogesa plant2. According to various estimates, injecting 100 m3 of coke oven gas per ton of pig iron can reduce the carbon consumption from coke by 30 kg per ton of pig iron.
Due to technical limitations, the use of hydrogen alone in a blast furnace is not feasible; therefore, its application within the blast furnace–converter route may only be regarded as an interim step toward the transition to direct reduced iron production3 [6 – 8].
Many studies have focused on the production of hydrogen-enriched gas through the gasification of various types of biomass [9 – 13], including charcoal, tar, hydrocarbons, wood, and synthetic natural gas. At small-scale enterprises, this approach is becoming one of the measures aimed at reducing CO2 emissions.
The reduction of iron ore materials by hydrogen-containing gases during pig iron production is associated with certain challenges [14 – 17]. Computational experiments make it possible to analyze the state of the system and the physicochemical processes involved, and, based on the resulting models, draw conclusions about the behavior of the substances under study.
The depletion of traditional iron ore reserves in the Urals, which have been exploited for over 300 years, poses a challenge for the ferrous metallurgy industry, prompting a transition to alternative types of iron ore. One such alternative is titanium-bearing ore, which contains, in addition to iron, vanadium and titanium. Its integrated processing – including the production of steel, vanadium pentoxide, pigment-grade titanium dioxide, and titanium sponge – represents a technologically and economically complex task that requires the optimization of the recovery processes for all valuable components. As demonstrated by the experience of the Institute of Metallurgy of the Ural Branch of the Russian Academy of Sciences, a promising solution lies in the use of information systems that describe the physicochemical and thermophysical processes occurring in metallurgical units. These systems enable the optimization of process parameters and enhance the recovery efficiency of target components – both of which are critical for the economic viability of titanium-bearing ore processing.
The aim of this study is to examine the thermodynamics of the reduction of iron ore titanomagnetite pellets in atmospheres of various gas mixtures, including those similar in composition to synthesis gas, blast furnace gas, coke oven gas, and other hydrogen-containing process gases viewed as promising for recycling applications.
Research methodology
This study employed the method of thermodynamic modeling based on the analysis of the equilibrium state of systems. The research was conducted using the Terra software package developed at Bauman Moscow State Technical University [18 – 20]. The advantages of this software include:
– the ability to define equilibrium conditions of a thermodynamic system with the environment using any pair of thermodynamic parameters (P – pressure, V – specific volume, T – temperature, S – entropy, H – enthalpy, and U – internal energy);
– the ability to perform equilibrium calculations for thermodynamic systems of arbitrary elemental composition;
– the inclusion of any individual substances in the expected composition of the system by adjusting the input data, and the determination of the equilibrium phase composition without the need to predefine thermodynamically permissible states;
– the option to exclude any substances from the equilibrium composition;
– the assignment of specific concentrations for substances, with the remaining composition calculated accordingly;
– the ability to account for the volume occupied by condensed phases, and more.
The material used in the study consisted of titanium-bearing iron ore pellets. The initial chemical composition of the pellets is presented in Table 1. The reduction modeling was carried out for atmospheres composed of CO – N2 – H2 – СН4 gas mixtures. The compositions of the reducing gases are shown in Table 2.
Table 1. Initial composition of the gas reducing system
Table 2. Compositions of reducing gases
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The thermodynamic database used for the calculations was compiled based on data from IVTANTHERMO and HSC, and includes thermodynamic property sets for both the initial components of the gas phase (CO, СН4 , H2 , N2 ) and the expected products of their interactions (CO2 , H2O and many others), as well as condensed carbon (graphite).
Two independent parameters were used: temperature (in the range of 493 – 1793 K, in 100 K increments) and pressure (0.1 MPa).
Результаты исследования и их обсуждение
The initial system for the reduction of pellets in an atmosphere of hydrogen-containing gases consists of a gas phase and condensed phases. The gas phase includes CO, N2 , H2 , and CH4 . The condensed phase consists of a metallic solution (s1) and an oxide solution (s2).
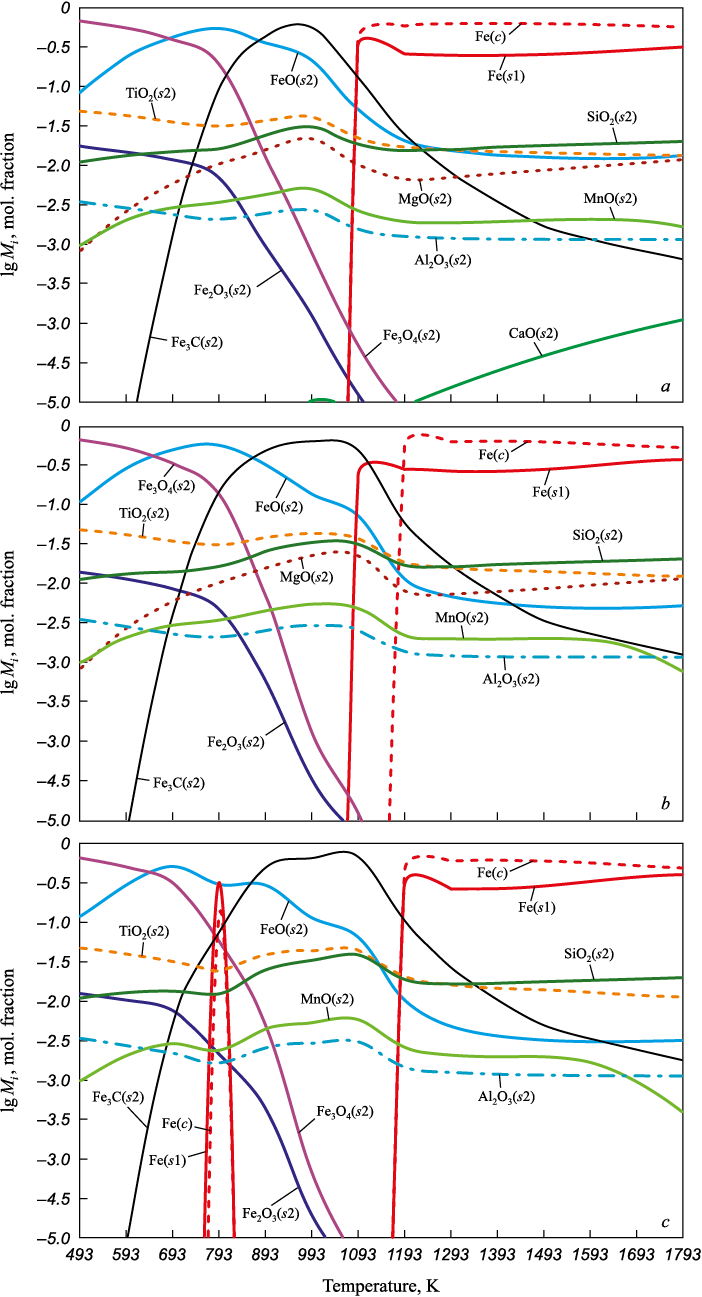
The composition of the condensed oxide phase is shown in Fig. 1.
Fig. 1. Composition of the condensed oxide phase during gas reduction: |
According to the graphical data, the most significant components are Fe(s1), FeO(s2), and Fe3O4(s2), each with a concentration exceeding 10–1 mol. fraction. In the temperature range of 1100 – 1793 K (Fig. 1, a), Fe(s1) becomes the dominant component, with a concentration of 0.88 mol. fraction. Under reduction in gas mixtures 2 and 3, Fe(s1) also becomes the predominant component in the temperature range of 1193 – 1793 K (Fig. 1, b, c).
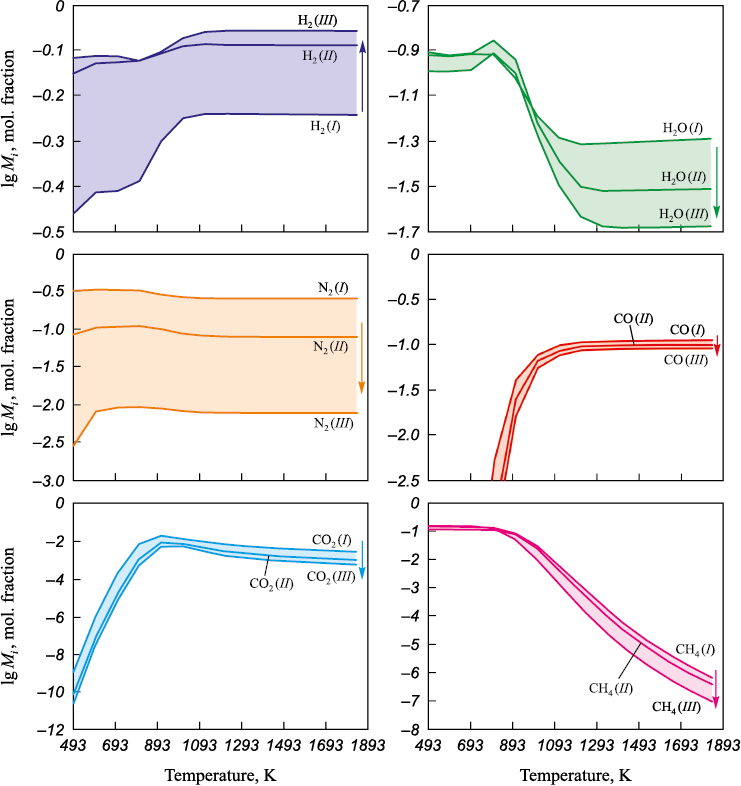
Changes in the composition of the gas phase are shown in Fig. 2. At temperatures above 900 K, the main components of the gas phase are H2 , N2 (p ~ 0.58 atm), and CO.
Fig. 2. Gas phase composition during reduction with different mixtures of gases (I, II, III) |
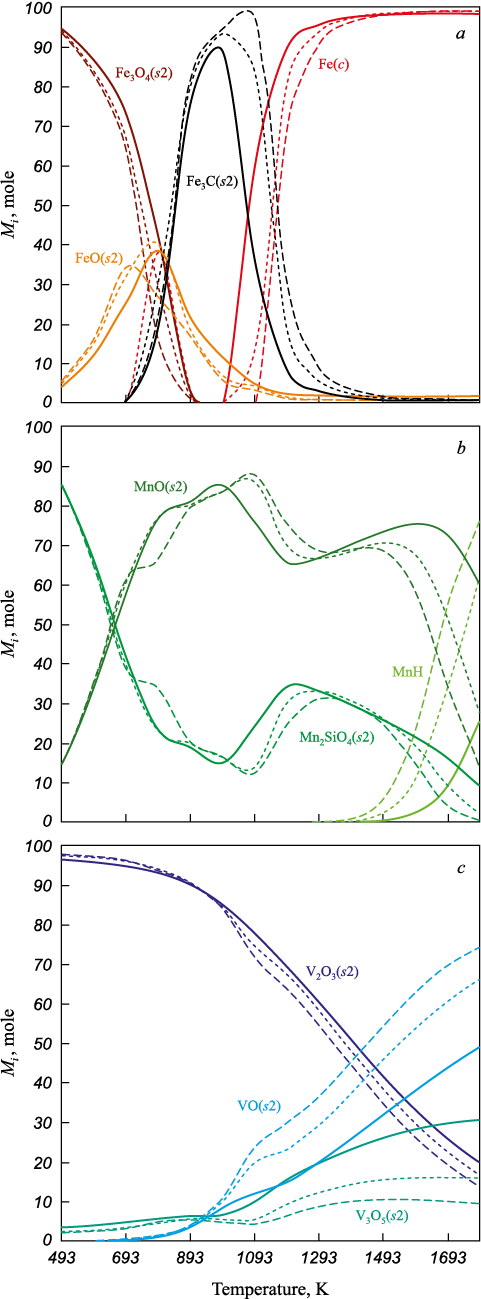
The phase distribution of iron, vanadium, and manganese as a function of temperature is presented in Fig. 3. In the temperature range of 500 – 893 K, the dominant iron phase is Fe3O4(s2) with a concentration of up to 86 mol. %. In the 593 – 993 K range, FeO(s2) in appears in concentrations ranging from 11 to 40 mol. %. At 993 K, the formation of iron carbide Fe3C(s2) occurs, reaching concentrations of 88, 94, and 95 mol. %, respectively. The formation of Fe3C(s2) is attributed to an increase in carbon content in the gas phase, which is confirmed by the rise in CO and CO2 concentrations. A further temperature increase to 1000 – 1793 K leads to the appearance of metallic iron Fe(s1), with concentrations ranging from 89 to 98 mol. %.
Fig. 3. Iron (a), manganese (b), vanadium (c) |
At 500 K, condensed manganese silicate Mn2SiO4(s2) dominates, accounting for approximately 85 mol. %. Between 593 and 1093 K, the concentration of Mn2SiO4(s2) decreases to 15, 13, and 12 mol. %, respectively, accompanied by an increase in condensed manganese oxide MnO(s2) from 34 to 86 mol. %. Further heating to 1700 K results in an increase in the concentration of gaseous manganese hydride MnH, reaching 9, 34, and 55 mol. %, respectively.
In the temperature range of 500 – 793 K, the dominant vanadium phase is condensed V2O3(s2), accounting for about 96 mol. %. As the temperature rises to 1700 K, the share of V2O3(s2) gradually decreases to 26, 22, and 19 mol. %. At the same time, an increase in the concentration of condensed vanadium oxide VO(s2) is observed above 893 K. Additionally, the concentration of condensed V3O5(s2) increases with temperature, reaching 29, 16, and 10 mol. % at 1700 K.
In the temperature range of 500 – 1493 K, the majority of silicon is present in the form of condensed magnesium silicate MgSiO3(s2), with a concentration ranging from 27 to 43 mol. %. The concentrations of calcium silicate CaSiO3(s2) (approximately 16 mol. %) and magnesium silicate Mg2SiO4(s2) (22 to 26 mol. %) remain nearly constant throughout the 500 – 1700 K range. A temperature increase to 1500 – 1700 K leads to a rise in the content of condensed silicon dioxide SiO2(s2) to 34 mol. %.
The investigation of the sample’s phase composition over the 500 – 1793 K temperature range revealed the following patterns. In the initial state (500 K), Al2O3(s2) is the dominant mineral phase of aluminum, accounting for 70 mol. %. Upon heating to 1700 K, the Al2O3(s2) content decreases to 58 mol. %, indicating the occurrence of phase transformations. Simultaneously, the concentration of MgAl2O4(s2) increases from 30 to 42 mol. %, pointing to the formation of a new phase. In the 500 – 1700 K range, the primary mineral phase of titanium is TiO2(s2). Between 993 and 1700 K, the concentration of TiO2(s2) decreases from 70 to 43 mol. %. The CaTiO3(s2) content remains stable at approximately 28 mol. % throughout the entire temperature interval. At temperatures above 1093 K, the concentrations of MgTi2O5(s2) and Mg2TiO4(s2) increase to 12 and 6 mol. %, respectively, indicating the formation of new mineral phases. The predominant magnesium phase throughout the 500 – 1793 K range is Mg2SiO4(s2), with a molar fraction of 43 – 54 mol. %. Heating to 1793 K leads to a decrease in MgSiO3(s2) content from 43 to 27 mol. %. A slight increase in the Mg2TiO4(s2) concentration to 6 mol. % is also observed. Between 500 and 1793 K, most of the calcium remains in the form of condensed CaSiO3(s2) (~49 – 55 mol. %), while the CaTiO3(s2) content (~45 mol. %) remains unchanged in all three cases.
Thus, increasing the temperature to 1793 K results in significant restructuring of the mineral composition due to reactions between various phases. Changes in the CO, N2 , and H2 content of the gas mixture do not affect the elemental distribution of silicon, aluminum, titanium, magnesium, and calcium. However, substantial changes are observed for iron, vanadium, and manganese.
In the temperature range of 500 – 893 K, the amount of condensed Fe3O4(s2) phase decreases, with gas mixture 3 showing a lower Fe3O4(s2) content than mixture 1. The maximum concentration of FeO(s2) in the system is approximately 40 mol. % at 793 K for gas mixtures 1 and 2, and 34 mol. % at 693 K for mixture 1. The maximum content of iron carbide Fe3C(s2) is about 96 mol. % at 1093 K in gas mixture 3, approximately 94 mol. % at 993 K in mixture 2, and ~88 mol. % in mixture 1. The formation of Fe(s1) begins at 993 K in mixtures 1 and 2, and at 1193 K in mixture 3. The amount of Fe(s1) formed in reducing gas mixture 1 is higher than in mixtures 2 and 3.
When the temperature exceeds 1093 K, the contents of condensed V2O3(s2) and V3O5(s2) are lower compared to those in gas mixture 1. The concentration of VO(s2) is highest in gas mixture 3.
The amounts of MnO(s2) and Mn2SiO4(s2) are greater between 1093 and 1393 K in mixture 3 compared to mixture 1; however, in the 1393 – 1793 K range, their concentrations increase in mixture 1. In addition, an increase in hydrogen content in the gas phase leads to a higher concentration of MnH.
Conclusions
The study of thermodynamic processes involved in the indirect reduction of titanomagnetite iron ore pellets using various gas atmospheres made it possible to identify patterns in the phase composition changes of the system depending on temperature and the composition of the reducing gas mixture.
Experimental results confirmed that in the temperature range of 500 – 1793 K, the equilibrium concentrations of silicon, aluminum, titanium, magnesium, and calcium remain virtually unchanged when different gas mixtures containing CO, N2 , H2 , and CH4 in varying proportions are used.
At the same time, significant changes in the concentrations of iron, vanadium, and manganese were observed depending on the composition of the gas mixture. For instance, in gas mixture 1 (20 % CO – 65 % N2 – 10 % H2 – 5 % СН4 ) a decrease in Fe3C(s2) content and an increase in Fe(s1), Fe3O4(s2), V2O3(s2), V3O5(s2), MnO(s2), and Mn2SiO4(s2) contents were observed in the 1393 – 1793 K temperature range.
In gas mixture 3 (50 % CO – 5 % N2 – 40 % H2 – 5 % СН4 ) a reduction in Fe3O4(s2) content and an increase in FeO(s2), Fe3C(s2), VO(s2), MnO(s2), and Mn2SiO4(s2) contents were noted in the 1093 – 1393 K temperature range.
The obtained results demonstrate the significant influence of the reducing gas mixture composition – particularly hydrogen content – on the phase equilibrium during the indirect reduction of iron ore pellets. This has important implications for the optimization of technological processes in iron production.
References
1. Digonskyii S.V., Ten V.V. Unknown Hydrogen: The Role of Hydrogen in the Polymorphism of Solids, Processes of Solid-Phase Reduction of Oxides and Sintering of Powders. St. Petersburg: Nauka; 2006:292. (In Russ.).
2. Morozova O.N., Pavlenko A.A., Titov S.S. Methods of hydrogen production. South Siberian Scientific Bulletin. 2019;(4(28)):188–194. (In Russ.). https://doi.org/10.25699/SSSB.2019.28.46373
3. Solodova N.L., Minigulov R.R., Emelyanycheva E.A. Hydrogen as a promising energy carrier. Modern methods of hydrogen production. Bulletin of Kazan Technological University. 2015;18(3):137–140. (In Russ.). https://doi.org/10.24412/Fg4yW5JCGyE
4. Ershov Yu.L., Shakurov A.G., Parshin V.M., Kolesnikov A.G., Shishov A.Yu. Hydrogen era in Russian metallurgy. Report 1. Steel in Translation. 2021;51(11):839–845. https://doi.org/10.3103/S0967091221110048
5. Ershov Yu.L., Shakurov A.G., Parshin V.M., Kolesnikov A.G., Shishov A.Yu. Hydrogen era in Russian metallurgy. Report 2. Steel in Translation. 2021;51(12):930–938. https://doi.org/10.3103/S0967091221120044
6. Gao X., Zhang R., You Zh., Yu W., Dang J., Bai Ch. Use of hydrogen-rich gas in blast furnace ironmaking of V-bearing titanomagnetite: Mass and energy balance calculations. Materials. 2022;15(17):6078. https://doi.org/10.3390/ma15176078
7. Okosun T., Nielson S., Zhou Ch. Blast furnace hydrogen injection: investigating impacts and feasibility with computational fluid dynamics. JOM. 2022;74:1521–1532. https://doi.org/10.1007/s11837-022-05177-4
8. Yu X., Hu Z., Shen Y. Modeling of hydrogen shaft injection in ironmaking blast furnaces. Fuel. 2021;302:121092. https://doi.org/10.1016/j.fuel.2021.121092
9. Suopajärvi H., Pongrácz E., Fabritius T. Bioreducer use in Finnish blast furnace ironmaking – Analysis of CO2 emission reduction potential and mitigation cost. Applied Energy. 2014;124:82–93. https://doi.org/10.1016/j.apenergy.2014.03.008
10. Liu Y., Shen Ya. Modelling and optimization of biomass injection in ironmaking blast furnaces. Progress in Energy and Combustion Science. 2021;87:100952. https://doi.org/10.1016/j.pecs.2021.100952
11. Luo S., Zhou Ya., Yi Ch. Hydrogen-rich gas production from biomass catalytic gasification using hot blast furnace slag as heat carrier and catalyst in moving-bed reactor. International Journal of Hydrogen Energy. 2012;37(20):15081–15085. https://doi.org/10.1016/j.ijhydene.2012.07.105
12. Xie H., Li R., Wang Zh., Yao X., Yu Q. Hydrogen production of bio-oil steam reforming combining heat recovery of blast furnace slag: Thermodynamic analysis. International Journal of Hydrogen Energy. 2019;44(47):25514–25523. https://doi.org/10.1016/j.ijhydene.2019.08.014
13. Feliciano-Bruzual C. Charcoal injection in blast furnaces (Bio-PCI): CO2 reduction potential and economic prospects. Journal of Materials Research and Technology. 2014; 3(3):233–243. https://doi.org/10.1016/j.jmrt.2014.06.001
14. Afanas’ev V.K., Gorlova S.N., Kuznetsova E.V., Sochnev A.V., Efanov G.I., Tolstoguzov V.N., Kuskov B.A. On the role of hydrogen in blast furnace process of iron production. Obrabotka metallov: tekhnologiya, oborudovanie, instrumenty. 2004;(4(25)):15–18. (In Russ.).
15. Rogozhnikov S.P., Rogozhnikov I.S. Determination of degree of hydrogen usage in blast furnace. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2020;75(10):1129–1134. (In Russ.). https://doi.org/10.32339/0135-5910-2019-10-1129-1134
16. Yalunin M.S., Vit’kina G.Yu., Dmitriev A.N., Zolotykh M.O., Alektorov R.V. Evaluation of the influence of reducing gas with an increased proportion of hydrogen on the efficiency of blast furnace smelting. In: Heat Engineering and Informatics in Education, Science and Production (TIM’2022). Yekaterinburg; 2022:185–190. (In Russ.).
17. Shevelev L.N. Assessment of economic, energy and ecological efficiency of iron and steel production from ore-coal briquettes in electric-furnace melting facility with application of hydrogen fuel. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2021;77(8):918–924. (In Russ.). https://doi.org/10.32339/0135-5910-2021-8-918-924
18. Vatolin N.A., Moiseev G.K., Trusov B.G. Thermodynamic Modeling in High-Temperature Inorganic Systems. Moscow: Metallurgy; 1994:352. (In Russ.).
19. Rybenko I.A., Protopopov E.V. Thermodynamic modeling of iron recovery processes. Izvestiya. Ferrous Metallurgy. 2021;64(11):825–831. (In Russ.). https://doi.org/10.17073/0368-0797-2021-11-825-831
20. Gamov P.A., Mal’kov N.V., Roshchin V.E. Thermodynamic modelling of the metals’ reduction process from the Suroyam titanomagnetite concentrate. Bulletin of the South Ural State University. Series: Metallurgy. 2018;18(2):21–28. (In Russ.). http://dx.doi.org/10.14529/met180203
About the Authors
A. N. DmitrievРоссия
Andrei N. Dmitriev, Dr. Sci. (Eng.), Prof., Chief Researcher of the Laboratory of Pyrometallurgy of Reduction Processes
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Yu. E. Burova
Россия
Yuliya E. Burova, Junior Researcher of the Laboratory of Pyrometallurgy of Reduction Processes
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
G. Yu. Vit’kina
Россия
Galina Yu. Vit’kina, Cand. Sci. (Eng.), Leading Researcher, Head of the Laboratory of Pyrometallurgy of Reduction Processes
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
N. M. Barbin
Россия
Nikolai M. Barbin, Dr. Sci. (Eng.), Chief Researcher of the Laboratory of Pyrometallurgy of Reduction Processes, Institute of Metallurgy, Ural Branch of the Russian Academy of Sciences; Prof., Ural Institute of State Fire Service of EMERCOM of Russia
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
22 Mira Str., Yekaterinburg 620062, Russian Federation
D. I. Terent’yev
Россия
Dmitrii I. Terent’yev, Cand. Sci. (Chem.), Leading Researcher
22 Mira Str., Yekaterinburg 620062, Russian Federation
Review
For citations:
Dmitriev A.N., Burova Yu.E., Vit’kina G.Yu., Barbin N.M., Terent’yev D.I. Thermodynamic modelling of reduction of iron ore materials by hydrogen-containing gases. Izvestiya. Ferrous Metallurgy. 2025;68(2):163-170. https://doi.org/10.17073/0368-0797-2025-2-163-170
JATS XML