Scroll to:
Effect of mechanical processing on reduction of iron oxides in man-made raw materials
https://doi.org/10.17073/0368-0797-2024-6-671-678
Abstract
The study considers ways to increase the efficiency of reduction of iron oxides from man-made waste (dust from electric arc furnaces) using mechanochemical activation (MCA), grinding and pressing. The analysis of chemical and phase compositions of the dust samples was carried out, which made it possible to identify their potential for processing. The experiments included a study of the effect of grinding and pressing at pressures up to 300 MPa on the materials’ phase composition, as well as an assessment of the effects of coke addition during MCA. To study the effect of pressing pressure on the reduction processes, briquettes were fired at a temperature of 1200 °C. The results showed that the degree of iron metallization increases with an increase in pressing pressure: concentration of metallic iron reaches 19 % at a pressure of 300 MPa, which is higher compared to 17 % in the initial state without pressing. The novelty of the work lies in optimizing the pressing parameters and demonstrating its effect on the iron reduction process. The proposed conditions make it possible to increase the efficiency of processing man-made waste, which can be used to improve the environmental and economic components of production.
Keywords
For citations:
Kleonovskii M.V., Sheshukov O.Yu., Mikheenkov M.A., Mikheenkov A.M., Matyukhin O.V. Effect of mechanical processing on reduction of iron oxides in man-made raw materials. Izvestiya. Ferrous Metallurgy. 2024;67(6):671-678. https://doi.org/10.17073/0368-0797-2024-6-671-678
Introduction
The processing of man-made raw materials has become one of the key challenges in modern industry and environmental management. Man-made raw materials include various wastes and by-products generated during industrial processes. These materials often contain valuable components, such as metals, minerals, and chemical compounds, which can be extracted and reused. The efficiency of raw material processing can be enhanced through grinding and pressing, i.e., mechanochemical activation (MCA) [1; 2].
Mechanochemical activation is a process in which mechanical action is applied to solid substances, leading to changes in their physicochemical properties. This action can involve operations such as grinding, pressing, rolling, or other forms of mechanical impact. The MCA process is employed to enhance material reactivity [3], modify phase composition [4], improve interactions between components, and activate chemical reactions [5] that would otherwise occur slowly or not at all under standard conditions.
Outlined below are the key aspects of MCA.
• Grinding and lattice destruction. During grinding, the crystalline lattice of solid substances is disrupted, leading to the formation of defects and an increase in the specific surface area. This enhances the material’s reactivity, as defects can serve as nucleation centers for new phases and initiate chemical reactions [6 – 8].
• Formation of active centers. Mechanical impact generates active centers on the particle surfaces, including free radicals, lattice defects, and surface irregularities. These active centers can trigger chemical reactions that would otherwise occur very slowly or require high temperatures and catalysts under normal conditions [9; 10].
• Phase composition changes. Mechanochemical activation can significantly alter a material’s phase composition. For example, new phases that were absent in the initial material may form, or existing phases may transform into more stable or reactive forms [11 – 13].
• Increased chemical activity. Mechanochemically activated materials often demonstrate heightened chemical activity. This enhanced reactivity can be leveraged to accelerate the reduction of metals from oxides, synthesize new compounds, or break down otherwise stable chemical bonds [14 – 16].
• Lower reaction temperatures. Mechanochemical activation enables many chemical reactions to occur at lower temperatures than would otherwise be necessary. This effect is attributed to the accumulation of mechanical energy within the material, which helps to overcome the reaction’s energy barrier [17; 18].
Thus, MCA is an important tool for controlling the physicochemical properties of materials, enabling the development of innovative technologies and processes.
In earlier experiments on the conditions of the pyrometallurgical reduction of oxide scale, it was found that increasing the pressing pressure of the scale during its preparation for firing from 0 to 300 MPa doubled its metallization degree during heating, while the onset temperature of metallization decreased by more than 40 °C [19]. It was hypothesized that the observed effects during the pyrometallurgical reduction of oxide scale result from the mechanochemical activation of iron oxides in the scale during pressing. Accordingly, the objective of this study is to confirm this hypothesis, optimize the pressing parameters, and demonstrate the impact of mechanical processing of raw materials on the iron reduction process.
Evaluation of franklinite decomposition potential during MCA
The effects of grinding and pressing pressure on the phase composition of electric arc furnace (EAF) dust were studied. To evaluate the influence of MCA on the phase composition of EAF dust, the tested dust samples were mixed to prepare an averaged sample, which was then ground for 2 min and pressed at pressures ranging from 0 to 300 MPa. The composition of the raw mixture in the first series and the processing conditions are presented in Table 1.
Table 1. Composition of raw material mixture
| |||||||||||||||||||||||||||||||||||||
In the second series, coke was added to the dust, and the raw mixture was subjected to MCA. The composition of the raw mixture in the second series and the processing conditions are shown in Table 2.
Table 2. Состав сырьевой смеси второй серии и режимы обработки
| |||||||||||||||||||||||||||||||||||||
The processed products were subjected to quantitative phase analysis.
Quantitative X-ray phase analysis was carried out using a STADI-P diffractometer (STOE, Germany). Data were collected with CuKα radiation (40 kV, 30 mA), a graphite monochromator, within a scattering angle range of 2θ = 10 ÷ 70°, with a step size of 0.02° and a dwell time of 2 s per step. The results were analyzed using the PDF-2 database (Release 2008 RDB 2.0804).
Assessment of MCA's impact on the phase composition of EAF dust
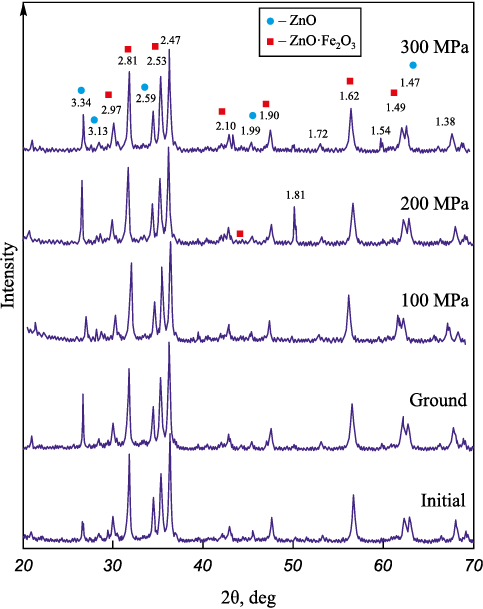
The phase analysis results for samples 1.1 – 1.5 without coke are presented in Fig. 1.
Fig. 1. Results of phase analysis of the samples 1.1 – 1.5 |
Analysis of the ground and pressed samples reveals that the intensity of the X-ray spectrum varies cyclically with changes in pressing pressure. Table 3 and Fig. 1 illustrate the variations in the phase composition of the samples under different processing conditions.
Table 3. Phase composition in the samples
| |||||||||||||||||||||||||||||||||
The findings indicate an inverse relationship in compound content. As the pressing pressure increases to 150 MPa, the ZnO content in the sample rises, while the franklinite (ZnO·Fe2O3 ) content decreases. Further increasing the pressing pressure to 300 MPa leads to a rise in franklinite (ZnO·Fe2O3 ) content and a corresponding decrease in ZnO content. Therefore, it is crucial to monitor and maintain an optimal pressing pressure to achieve the desired compound composition in the final product.
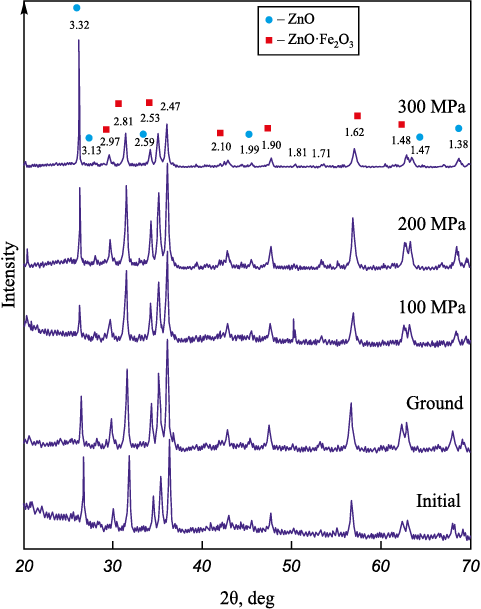
Phase analysis results for samples 2.1 – 2.5 with coke are presented in Fig. 2.
Fig. 2. Results of phase analysis of the samples 2.1 – 2.5 |
Analysis of the phase composition of the ground and pressed samples indicates that, similar to the samples without coke, the intensity of the entire X-ray spectrum changes cyclically depending on the pressing pressure. Table 4 and Fig. 2 present the variations in phase composition of the samples under different processing conditions.
Table 4. Phase composition in the samples 2.1 – 2.5
| |||||||||||||||||||||||||||||||||
The test results indicate that as the pressing pressure increases, the content of free ZnO initially decreases and then sharply rises. In the initial sample, the phase ratio of ZnO/ZnO·Fe2O3 is 37.4/40.7, whereas after complete MCA, this ratio shifts to 46.6/31.6. This change in phase quantities is likely due to interaction with coke according to the reaction
3ZnFe2O4 + C = Fe3O4 + 3ZnO + CO↑.(1)
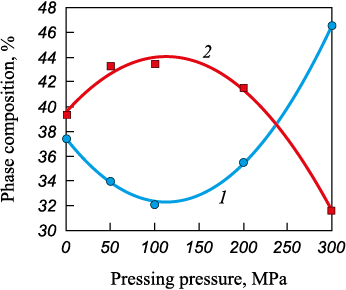
Fig. 3 shows the dependence of ZnO and ZnO·Fe2O3 phase content on pressing pressure.
Fig. 3. Phase composition in the samples 2.1 – 2.5 depending on processing modes: |
Firing of pressed samples
To evaluate the effect of pressing pressure on the phase composition of fired products, a raw mixture was prepared using electric arc furnace (EAF) dust, coke, and a dry binder component with a content of 10 %. The components of the raw mixture were co-ground. After grinding, a liquid binder component was added to the raw mixture, which was then briquetted under pressures of 0, 100, 200 and 300 MPa. The composition of the binder is provided in the patent [20], while the composition of the raw mixture for firing and its processing conditions are listed in Table 5.
Table 5. Composition of the raw mixture for firing and its processing mode
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Before briquetting, a binder was introduced, consisting of ladle furnace slag (LFS), liquid glass, and hydrofluorosilicic acid (HFSA). The LFS contains approximately 40 % dicalcium silicate (2CaOSiO2 ), which reacts with liquid glass, causing it to harden about 30 min after mixing and forming water-resistant tobermorite-like calcium-sodium hydrosilicates. This time is sufficient for briquetting to be carried out. Once briquetting and complete hardening are achieved, the briquettes gain high strength. Hydrofluorosilicic acid also reacts with liquid glass, promoting its hardening [21]. Additionally, the acid acts as a fluxing additive. It reacts with calcium oxide in the slag to form fluorite (fluorspar), which is a strong flux.
In [22], it was shown that when the binder content is less than 10 %, a non-diffusion reduction mode of iron oxides is implemented. In this mode, the degree of metallization is highly dependent on pressing pressure. When the binder content reaches 10 %, a liquid phase appears, and a diffusion reduction mode of iron oxides occurs. In this mode, the degree of metallization does not depend on pressing pressure and remains approximately the same across the entire pressure range.
The coke content corresponds to the stoichiometry of iron oxides and carbon, plus an additional 15 % to account for the ash content of the coke.
Dry briquettes were fired at temperatures up to 1200 °C for 1 h. The firing temperature matched the conditions for completing the metallization process [22]. An isothermal holding period of 30 min was maintained at 1200 °C. The overall appearance of the fired samples is shown in Fig.
The fired samples in Fig. 4 clearly show droplets of metallic iron.
Fig. 4. General view of the fired samples |
The firing products were subjected to phase analysis, and the phase content of the studied samples is presented in Table 6.
Table 6. Phase composition in the studied samples
| ||||||||||||||||||||
The test results indicate that as the pressing pressure increases, the metallic iron content initially decreases but then rises, reaching 19 % at 300 MPa compared to 17 % in the initial state without pressing. Based on these findings, it is recommended to maintain a pressing pressure of 300 MPa, as lower pressures may negatively impact the degree of metallization.
Results and discussion
The research demonstrated a significant influence of pressing pressure on the phase composition and reduction processes of iron oxides in ASF dust. In the first series of experiments, conducted on samples without coke addition, cyclic variations in phase content were observed depending on the pressing pressure (Table 3). At pressures up to 150 MPa, the ZnO content increased, while the content of franklinite (ZnO·Fe2O3) decreased. However, at higher pressures up to 300 MPa, the opposite effect was noted: ZnO content decreased, and franklinite content increased. These findings highlight the necessity of controlling pressing pressure to achieve the desired phase ratio in the final product.
In the second series of experiments, involving samples with coke addition (Table 4), phase analysis results similarly demonstrated cyclic variations in the contents of ZnO and ZnO·Fe2O3 depending on pressing pressure. A substantial increase in free ZnO content at 300 MPa indicates the occurrence of the reduction reaction of franklinite (ZnFe2O4 ) involving carbon. The interaction, described by Equation (1), results in the formation of magnetite (Fe3O4 ), zinc oxide (ZnO), and carbon monoxide (CO), confirming the role of MCA in the breakdown of franklinite and the reduction of iron oxides.
The results of evaluating the effect of pressing pressure on firing processes (Table 6) showed that as pressure increased from 0 to 300 MPa, the metallic iron content initially decreased but subsequently increased, reaching a maximum of 19 % at 300 MPa. These findings indicate that the optimal pressing pressure for maximizing iron metallization is 300 MPa. Lower pressures may adversely affect the reduction process, reducing the proportion of metallic iron in the final product.
Thus, the study confirms that MCA occurring during the pressing of EAF dust enhances the reduction processes of iron oxides. Optimizing pressing parameters, particularly maintaining a pressure of 300 MPa, ensures the highest metallization efficiency. This optimization offers promising opportunities to improve the productivity and environmental performance of pyrometallurgical waste processing.
Conclusions
It has been demonstrated that MCA significantly influences the phase composition of EAF dust, both with and without coke addition. In samples without coke, the phase composition changes cyclically with variations in pressing pressure. At pressures up to 150 MPa, the zinc oxide (ZnO) content increases, while the franklinite (ZnO·Fe2O3) content decreases. However, with further pressure increases to 300 MPa, the franklinite content rises, and the free ZnO content decreases, indicating the need for precise pressure control to achieve the desired phase composition.
In samples with coke addition, a similar cyclic change in phase composition is observed depending on the pressing pressure. As the pressure increases, the free ZnO content initially decreases but then sharply rises. These changes are likely attributed to the reaction between franklinite and coke, resulting in the formation of magnetite (Fe3O4 ), ZnO, and carbon monoxide.
Firing of pressed samples revealed that as the pressing pressure increases, the metallic iron content initially decreases but subsequently rises, reaching a maximum at 300 MPa. This suggests that a pressing pressure of 300 MPa is optimal for achieving a high degree of metallization, while lower pressures may negatively affect the quality of the final product.
References
1. Saedi A., Jamshidi-Zanjani A., Mohseni M., Darban A., Nejati H. Mechanical activation of lead–zinc mine tailings as a substitution for cement in concrete construction. Construction and Building Materials. 2023;364:129973. https://doi.org/10.1016/j.conbuildmat.2022.129973
2. Yuan W., Wu Z., Song Q., Huang Q., Zhang C., Crittenden J. Lead recovery from waste CRT funnel glass by mechanochemical reaction with reductive Al powder. Waste Management. 2023;172:43–50. https://doi.org/10.1016/j.wasman.2023.09.008
3. Mañosa J., Alvarez-Coscojuela A., Marco-Gibert J., Maldonado-Alameda A., Chimenos J.M. Enhancing reactivity in muscovitic clays: Mechanical activation as a sustainable alternative to thermal activation for cement production. Applied Clay Science. 2024;250:107266. https://doi.org/10.1016/j.clay.2024.107266
4. Penczner S., Kumar P., Patel M., Bouchard L., Iacopino D., Patel R. Innovations in mechanochemical synthesis: Luminescent materials and their applications. Materials Today Chemistry. 2024;38:102177. https://doi.org/10.1016/j.mtchem.2024.102177
5. Baki V.A., Ke X., Heath A., Calabria-Holley J., Terzi C., Sirin M. The impact of mechanochemical activation on the physicochemical properties and pozzolanic reactivity of kaolinite, muscovite and montmorillonite. Cement and Concrete Research. 2022;162:106962. https://doi.org/10.1016/j.cemconres.2022.106962
6. Kohobhange S.P., Manoratne C.H., Pitawala H.M., Rajapaks R.M. The effect of prolonged milling time on comminution of quartz. Powder Technology. 2018;330:266–274. https://doi.org/10.1016/j.powtec.2018.02.033
7. Yang X., Liu J., Chen G., Wu F., Liu J., Jiang X. Synergistic effects of mechanochemical activation and selective O-alkylation on the chemical structure and gaseous products evolution during coal flash pyrolysis. Journal of Analytical and Applied Pyrolysis. 2024;180:106553. https://doi.org/10.1016/j.jaap.2024.106553
8. Abed F.H., Zareei S.A., Kurdi N.H., Emami A. Enhancing geopolymer binder reactivity and performance via mechanochemical activation: A comprehensive study of rheological, mechanical, and microstructural properties. Construction and Building Materials. 2024;430:136456. https://doi.org/10.1016/j.conbuildmat.2024.136456
9. Yang C., Li Y., Tian Z., Qin W., Liu X., Wang X. Enhanced chalcopyrite leaching by mechanical activation: New insights from microstructure. Minerals Engineering. 2024; 212:108719. https://doi.org/10.1016/j.mineng.2024.108719
10. Hosseini S., Brake N.A., Nikookar M., Günaydın-Şen Ö., Snyder H.A. Enhanced strength and microstructure of dredged clay sediment-fly ash geopolymer by mechanochemical activation. Construction and Building Materials. 2021;301:123984. https://doi.org/10.1016/j.conbuildmat.2021.123984
11. Singh A., Bhadauria S., Thakare A., Kumar A., Mudgal M., Chaudhary S. Durability assessment of mechanochemically activated geopolymer concrete with a low molarity alkali solution. Case Studies in Construction Materials. 2024;20:e02715. https://doi.org/10.1016/j.cscm.2023.e02715
12. Zhihan Z., Zhi W., Dong W., Yong L., Wanhai X., Chenghao L., Yang L., Jian W., Guobiao L. A green process for selective REEs recovery from Rare earth waste through mechanochemical activation. Separation and Purification Technology. 2023;315:123654. https://doi.org/10.1016/j.seppur.2023.123654
13. Zhang Y., Liu B., Gu X., Nehdi M.L., Zhang L.V. Mechanochemical activation of iron ore tailing-based ternary supplementary cementitious materials. Construction and Building Materials. 2022;346:128420. https://doi.org/10.1016/j.conbuildmat.2022.128420
14. Odebiyi O.S., Guo Y., Hao Du, Liu B., Wang S. Effect of mechanochemical activation parameters on vanadium recovery from vanadium-bearing steel slag: Critical speed derivation for wet-ball milling. Materials Chemistry and Physics. 2024;324:129697. https://doi.org/10.1016/j.matchemphys.2024.129697
15. Yu Y., Cui L., Zhang L., Wang Y. Efficient mechanochemical leaching of zinc from zinc oxide ores. Transactions of Nonferrous Metals Society of China. 2024;34:1976–1993. https://doi.org/10.1016/S1003-6326(24)66520-9
16. Meng X., Hao J., Cao H., Lin X., Ning P., Zheng X., Chang J., Zhang X., Wang B., Sun Z. Recycling of LiNi1/3Co1/3Mn1/3O2 cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering. Waste Management. 2019;84:54–63. https://doi.org/10.1016/j.wasman.2018.11.034
17. Kriskova L., Pontikes Y., Cizer Ö., Mertens G., Veuleman W., Geysen D., Jones P.T., Vandewalle L., Van Balen K., Blanpain B. Effect of mechanical activation on the hydraulic properties of stainless steel slags. Cement and Concrete Research. 2012;42(6):778–788. https://doi.org/10.1016/j.cemconres.2012.02.016
18. Gao P., Wu X., Zhang D., Sun X., Zhang G., Chen F. Mechanochemical activation of aryl diazonium salts: Synthesis of polycyclic (hetero)aromatics. Journal of Organic Chemistry. 2024;89(17):12197–12203. https://doi.org/10.1021/acs.joc.4c01107
19. Sheshukov O.Yu., Mikheenkov M.A., Nekrasov I.V., Egiazar’yan D.V., Vedmid’ L.B. Influence of pressing pressure on reduction of iron oxides of technogenic origin. In: Proceedings of the Sci. and Pract. Conf. with Int. Part. and Elements of the School of Young Scientists: Dedicated to the 65th Anniversary of IMET UB RAS. 2020:472–474. (In Russ.).
20. Vedmid’ L.B. Mikheenkov M.A., Sheshukov O.Yu., Nekrasov I.V. Method of briquetting iron-containing waste in the form of scale. Patent RF no. 2705483. MPK C22B1/245. Bulleten’ izobretenii. 2019;(31). (In Russ.).
21. Borsuk P.A., Lyass A.M. Liquid Self-Hardening Mixtures. Moscow: Mashinostroenie; 1979:255. (In Russ.).
22. Sheshukov O., Mikheenkov M., Vedmid L., Nekrasov I., Egiazaryan D. Mechanism of ion-diffusion solid-phase reduction of iron oxides of technogenic origin in the presence of the liquid phase and without it. Metals. 2020;10(12):1564. https://doi.org/10.3390/met10121564
23.
About the Authors
M. V. KleonovskiiRussian Federation
Mikhail V. Kleonovskii, Engineer of the Chair “Metallurgy of Iron and Alloys”
19 Mira Str., Yekaterinburg 620002, Russian Federation
O. Yu. Sheshukov
Russian Federation
Oleg Yu. Sheshukov, Dr. Sci. (Eng.), Prof., Director of the Institute of New Materials and Technologies, Ural Federal University named after the first President of Russia B.N. Yeltsin; Chief Researcher of the Laboratory of Powder, Composite and Nano-Materials, Institute of Metallurgy, Ural Branch of the Russian Academy of Sciences
19 Mira Str., Yekaterinburg 620002, Russian Federation
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
M. A. Mikheenkov
Russian Federation
Mikhail A. Mikheenkov, Dr. Sci. (Eng.), Prof., Ural Federal University named after the first President of Russia B.N. Yeltsin; Senior Researcher of the Laboratory “Pyrometallurgy of Ferrous Metals”, Institute of Metallurgy, Ural Branch of the Russian Academy of Sciences
19 Mira Str., Yekaterinburg 620002, Russian Federation
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. M. Mikheenkov
Russian Federation
Aleksandr M. Mikheenkov, Postgraduate of the Chair “Metallurgy of Iron and Alloys”
19 Mira Str., Yekaterinburg 620002, Russian Federation
O. V. Matyukhin
Russian Federation
Oleg V. Matyukhin, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Thermal Physics and Informatics in Metallurgy”
19 Mira Str., Yekaterinburg 620002, Russian Federation
Review
For citations:
Kleonovskii M.V., Sheshukov O.Yu., Mikheenkov M.A., Mikheenkov A.M., Matyukhin O.V. Effect of mechanical processing on reduction of iron oxides in man-made raw materials. Izvestiya. Ferrous Metallurgy. 2024;67(6):671-678. https://doi.org/10.17073/0368-0797-2024-6-671-678