Scroll to:
Thermodynamic modeling of converter sludge sintering
https://doi.org/10.17073/0368-0797-2024-6-725-730
Abstract
Currently, a promising area is the development of technologies for sintering or briquetting of converter sludge. Recycling of this sludge into production will allow solving a number of important tasks for modern metallurgy in the utilization of man-made waste, saving raw materials and reducing the cost of steel. The efficiency of utilizing useful components in the composition of briquettes is significantly higher than in any other state (in a fine or polydisperse fraction, in sorted form). In this paper, we consider the development and justification of an integrated approach to thermochemical sintering of converter sludge based on conditioning of iron-containing sludge by non-thermal adsorption dehydration and thermochemical sintering with simultaneous reduction of iron from oxides. Adsorption dehydration to a moisture content of 2 – 3 % is provided by a short-term contact of iron-containing slimes with a porous energy carrier, brown coal semi-coke, which is separated by pneumoseparation and sent for energy technological use, and the iron-containing product mixed with coals is subjected to thermo-oxidative coking. Coking is carried out in an annular furnace with a rotating hearth, where, when temperatures reach 1050 – 1100 °C, a large and durable lump material is formed with 55 – 60 % of the iron-containing product with almost complete reduction. Thermodynamic modeling of converter sludge sintering with coals was carried out. A tool for performing computational experiments using methods of thermodynamic modeling of the studied object was the Terra software package designed to calculate the thermodynamic properties and composition of the phases of equilibrium state of arbitrary systems with chemical and phase transformations. The results of thermodynamic modeling were fully confirmed by the experimental studies. The obtained material is an analog of ferrocox containing 35 – 39 % of iron and 45 – 49 % of carbon, while the zinc oxide content does not exceed 0.017 %.
Keywords
For citations:
Protopopov E.V., Rybenko I.A., Belenetskii E.A. Thermodynamic modeling of converter sludge sintering. Izvestiya. Ferrous Metallurgy. 2024;67(6):725-730. https://doi.org/10.17073/0368-0797-2024-6-725-730
Introduction
In modern practices, the oxygen-converter process plays a dominant role in global steelmaking [1 – 4]. The development and refinement of converter unit designs, coupled with accumulated knowledge on the use of combined melt blowing, have significantly enhanced the versatility of the converter process. This includes smelting technologies involving liquid-phase reduction of industrial waste and the use of multipurpose additives or briquettes [5 – 8].
Studies [9 – 12] indicate that steel production in oxygen converters generates approximately 12 – 25 kg of fine dust per ton of steel, which is a valuable iron-containing industrial by-product. For example, converter sludge from EVRAZ United West Siberian Metallurgical Plant JSC (EVRAZ ZSMK) contains up to 57 – 63 % Fe2O3 and 46.8 % total Fe [13]. Recycling this sludge back into production addresses critical metallurgical challenges, including waste utilization, raw material conservation, and steel production cost reduction [14 – 15]. However, despite the clear potential of converter sludge recycling, its direct introduction into the charge of oxygen converters or blast furnaces in fine-dispersed form is infeasible.
Iron-containing materials for the blast furnace or converter are typically introduced in lump form. Therefore, industrial waste (e.g., mill scale, dust, and dewatered sludge) is traditionally utilized, for instance, by adding it to charge [13]. However, introducing fine-particle materials into the charge in significant quantities is generally accompanied by a decrease in process productivity and a deterioration in the strength characteristics of the final sinter [14].
For this reason, the development of technologies for sintering or briquetting converter sludge remains a promising direction. Briquetting has several advantages: it enables the conversion of industrial waste with diverse chemical compositions and properties into standard products with controlled fraction sizes and technological characteristics. This increases the density of the composite material, prevents caking and blockages of fine waste in hoppers and dosing equipment, and reduces dust during transportation and use [14]. Moreover, the efficiency of utilizing valuable components in briquettes is significantly higher than in fine or polydisperse fractions, sorted forms, or other states.
Thus, briquetting converter sludge for subsequent recycling has distinct advantages over its use in sinter mix. However, the sludge must first undergo dehydration. Currently, various sludge dehydration methods exist, but they are typically bulky, complex, and energy-intensive, involving preliminary mechanical moisture removal (to below 20 – 25 %) through thickening or filtration, followed by thermal drying [14].
This paper proposes a new integrated approach to the thermochemical sintering of converter sludge, based on conditioning iron-containing sludge through non-thermal adsorption dehydration and thermochemical sintering with simultaneous reduction of iron from oxides.
Adsorption dehydration to a moisture content of 2 – 3 % is achieved by short-term contact between iron-containing sludge and a porous energy carrier – brown coal semi-coke (BCSC). After dehydration, BCSC is separated by pneumoseparation and directed for energy-technological use, while the iron-containing product, mixed with coal (grades GZh or Zh), undergoes thermo-oxidative coking in an annular furnace with a rotating hearth. At temperatures of 1050 – 1100 °C, a large, durable lump material is formed containing 55 – 60 % iron with almost complete reduction.
Brown coal semi-coke is a relatively new product for metallurgy, but numerous laboratory and industrial studies have demonstrated its effectiveness in pig iron and steel production, thermal energy generation, and recycling of high-moisture waste. In this case, BCSC is a low-ash, low-sulfur product with high energy potential and increased reactivity and adsorption capacity, making it suitable for preliminary dehydration of converter sludge [15; 16]. After preliminary dehydration, the proposed technological scheme suggests a thermochemical sintering method in a mixture with sinterable coals in an annular furnace with a rotating hearth.
Research methods
To address optimization tasks, thermodynamic modeling of the converter sludge sintering process with coals was conducted. The Terra software package, developed at Bauman Moscow State Technical University, was selected as the tool for computational experiments using thermodynamic modeling methods for the studied system. This software is designed to calculate the thermodynamic properties and phase composition of equilibrium states in arbitrary systems with chemical and phase transformations [17; 18]. The program demonstrates consistently good convergence when modeling processes in elementary systems, including the direct reduction of metals in complex multicomponent heterogeneous systems [19; 20].
Computational experiments were conducted for two types of mixtures:
– 50 % Kuznetsk enrichment plant concentrate (coal grades Zh and GZh) and 50 % converter sludge;
– 50 % concentrate of grade Zh coal from the Mezhegey deposit and 50 % converter sludge.
The composition of the converter sludge is as follows (wt. %): Fe2O3 64.05; FeO 1.82; MgO 4.59; CaO 16.68; SiO2 5.75; K2O 0.19; V2O5 0.07; Cr2O3 0,10; C 0.63; S 0.24; ZnO 1.11; CuO 0.06; PbO 0.11; MnO 1.08; Al2O3 1.93; Na2O 0.88; P2O5 0.32; TiO2 0.21; W 1.35. The table below presents the characteristics of the coal concentrates, where Wr is moisture content; Аd is ash content; Vdaf is volatile matter yield; Sd is sulfur content [14].
Characteristics of coal concentrates
|
Results and discussion
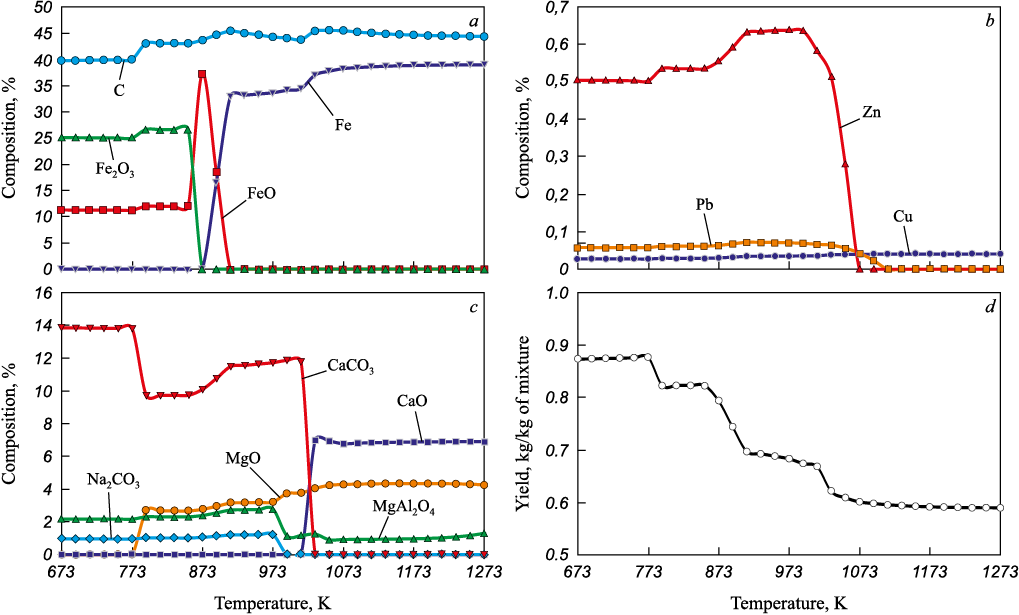
The results of the thermodynamic modeling (Fig. 1) were nearly identical for the tested conditions and demonstrated that the reduction of iron begins at a temperature of approximately 873 K. At temperatures above 1073 K, the chemical composition of the briquettes stabilizes. The iron content reaches a maximum of approximately 39 %, and the carbon content is 45 %. Additionally, the semi-product contains, (wt. %): CaO 6.9, MgO 4.3, MgAl2O4 1.0. The briquette mass amounts to 0.6 kg per kg of the initial mixture.
Fig. 1. Results of thermodynamic modeling of converter sludge sintering with coal |
The zinc and lead content drops to nearly zero at temperatures above 1073 K, as compounds of these elements presumably transition to the gas phase. Copper content remains at approximately 0.04 %. Other elements (titanium, chromium, vanadium, sodium, potassium) are present in the system in trace amounts as oxides (less than 0.1 %).
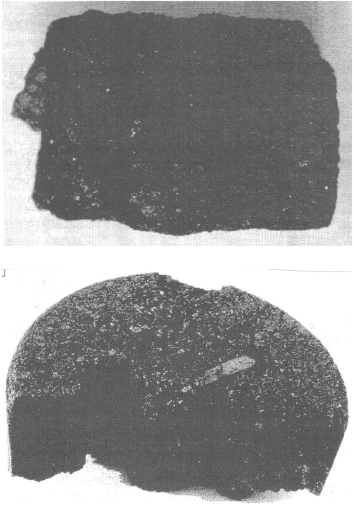
This information is fully supported by experimental studies, where the mixtures were heated in an annular furnace to a temperature of 1003 K and then in a Tamman furnace for 30 min at the final process temperature of 1373 K. The data indicate that the briquetted material resembles ferrocoke (Fig. 2), containing 35 – 39 wt. % Fe and 45 – 49 wt. % C, while the zinc oxide content does not exceed 0.017 wt. %.
Fig. 2. Experimental samples of ferrocox briquettes |
Further studies using the Terra software investigated the parameters of the semi-product at various ratios of converter sludge to coal in the composite charge within a temperature range of 873 – 1273 K. The results (Fig. 3) indicate that the carbon content in the product stabilizes at temperatures above 1073 K, reaching 50, 45 and 27 % for converter sludge proportions of 40, 50 and 60 % in the charge, respectively. The iron content is 26.5 % for a sludge proportion of 40 % in the charge and remains nearly constant at approximately 39 – 40 % for sludge proportions of 50 and 60 % at temperatures above 1073 K. Similar trends are observed for the yield of ferrocoke-type briquettes as a function of temperature at different ratios of charge components. The yield of the semi-product at the final reduction temperatures is 0.6 kg per kg of charge for a sludge proportion of up to 40 %. For sludge proportions of 50 and 60 %, the product yield shows only slight variation, ranging between 0.67 and 0.69 kg per kg of charge.
Fig. 3. Results of the study of converter sludge sintering |
Thus, the optimal ratio of converter sludge to charge for achieving the desired yield and composition of the semi-product should be 1:1. Increasing the proportion of sludge in the charge results in only minor changes to the amount of reduced iron and product yield; however, it leads to a decrease in carbon content in the semi-product.
The practical application of the resulting briquettes lies in their use as additives to the charge in the converter process, serving as an iron-containing material, an additional heat carrier, and a reducing agent.
Conclusions
The issues of thermochemical sintering of converter sludge with simultaneous iron reduction from oxides were analyzed. Thermodynamic and physical modeling of the processes of sludge sintering with various coals allowed us to consider the resulting material as an effective heat carrier and reducing agent for converter smelting. The rational composition of the initial charge for composite briquettes of the ferrocox type was determined.
References
1. Budanov I.A., Ustinov V.S. Prospects for development of metallurgical production in Russia. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2014;(5(1373)):3–12. (In Russ.).
2. Grigorovich K.V. Current state of ferrous metallurgy and directions of its development in digital economy. In: Proceedings of the XV Int. Congress of Steelmakers and Metal Manufacturers. 2018:42–59. (In Russ.).
3. Brun L.C. Overcapacity in Steel: China’s Role in Global Problem. Center of Globalization, Governance & Competitiveness, Duke University; 2016:54. https://doi.org/10.13140/RG.2.2.11923.48161
4. Osaba E., Carballedo R., Diaz F., Perallos A. Simulation tool based on a memetic algorithm to solve a real instance of a dynamic TSP. In: Proceedings of IASTED Int. Conf. of Applied Simulation and Modelling. 2012:27–33. https://doi.org/10.2316/P.2012.776-029
5. Sakthivel R., Vasumathi N., Sahu D., Mishra B.K. Synthesis of magnetite powder from iron ore tailings. Powder Technology. 2010;201(2):187–190. https://doi.org/10.1016/j.powtec.2010.03.005
6. Sanakulov K. Scientific-Technical Basis of Processing Mining-Metallurgical Wastes. Tashkent: FAN; 2009:404.
7. Yusupkhodjayev A.A. Theory Waste Free Technology on the Ferrous Metallurgy. Tashkent: TSTU; 2017:4.
8. Jiabin C., Wenlong J., Lianghui Y. Survey and evaluation of the iron tailings resources in China. Mineral Resources Development. 2010;(3):60–62.
9. Yusupkhodzhaev A.A., Valiev Kh.R., Khudoyarov S.R., Matkarimov S.T. Efficiency rise of steel making production via additional recovery of valuable components from utilized slags. Chernye metally. 2015;(1):19–22.
10. Chong Y.T., Teo K.M., Tang L.C. A lifecycle-based sustainability indicator framework for waste-to-energy systems and a proposed metric of sustainability. Renewable and Sustainable Energy Reviews. 2016;56:797–809. https://doi.org/10.1016/j.rser.2015.11.036
11. Su F., Lampinen H.-O., Robinson R. Recycling of sludge and dust to the BOF converter by cold bonded pelletizing. ISIJ International. 2004;44(4):770–776. https://doi.org/10.2355/isijinternational.44.770
12. Matsubae-Yokoyama K., Kubo H., Nagasaka T. Recycling effects of residual slag after magnetic separation for phosphorus recovery from hot metal dephosphorization slag. ISIJ International. 2009;95(3):306–312. https://doi.org/10.2355/tetsutohagane.95.306
13. Volynkina E.P., Kuznetsov S.N., Protopopov E.V., etc. Metallurgical Technologies for Processing Technogenic Deposits of Industrial and Household Waste. Novosibirsk: Publishing House of the SB RAS; 2014:294. (In Russ.).
14. Kuznetsov S.N., Shkoller M.B., Protopopov E.V., Temlyantsev M.V., Feiler S.V. Technological basics of adsorption dehydration and thermochemical sintering of BOF sludge. Izvestiya. Ferrous Metallurgy. 2017;60(4): 268–275. (In Russ.). https://doi.org/10.17073/0368-0797-2017-4-268-275
15. Shkoller M.B. Research and development of a method for recycling high-moisture and high-ash coal sludge. In: Modeling and High-Tech Information Technologies in Technical and Socio-Economic Systems. Proceedings of the V Int. Sci. and Pract. Conf. Novokuznetsk: IC SibSIU; 2021:213–217. (In Russ.).
16. Shkoler M.B. Theory and Practice of Using Fine-Grained Semi-Coke from Brown Coals to Produce Various Grades of Coke, Process Fuels, Composites and Solid Waste Recycling. Role: Open Science Publishing; 2019:134. (In Russ.).
17. Belov G.V., Trusov B.G. Thermodynamic Modeling of Chemically Reacting Systems. Moscow: Bauman MSTU; 2013;96. (In Russ.).
18. Belov G.V. Thermodynamic Modeling: Methods, Algorithms, Programs. Moscow: Nauchnyi Mir; 2002:184. (In Russ.).
19. Rybenko I.A. Thermodynamic Modeling of Processes in Elementary Systems. Novokuznetsk: SibSIU; 2016:97. (In Russ.).
20. Rybenko I.A. Development of optimal technological modes for the production of metals using mathematical modeling methods and instrumental systems. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2018;(2):57–61. (In Russ.).
About the Authors
E. V. ProtopopovRussian Federation
Evgenii V. Protopopov, Dr. Sci. (Eng.), Prof. of the Chair of Ferrous Metallurgy and Chemical Technology
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
I. A. Rybenko
Russian Federation
Inna A. Rybenko, Dr. Sci. (Eng.), Prof., Head of the Chair of Applied Information Technologies and Programming
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
E. A. Belenetskii
Russian Federation
Evgenii A. Belenetskii, MA Student of the Chair of Ferrous Metallurgy and Chemical Technology
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
Review
For citations:
Protopopov E.V., Rybenko I.A., Belenetskii E.A. Thermodynamic modeling of converter sludge sintering. Izvestiya. Ferrous Metallurgy. 2024;67(6):725-730. https://doi.org/10.17073/0368-0797-2024-6-725-730