Scroll to:
Thermodynamic modeling of cobalt and nickel reduction using hydrometallurgical enrichment concentrates for steel alloying
https://doi.org/10.17073/0368-0797-2024-4-384-390
Abstract
The article provides studies on reduction processes in model thermodynamic systems and processes of nickel reduction from nickel concentrate and cobalt and nickel from cobalt-nickel concentrate. Concentrates are obtained during hydrometallurgical enrichment of polymetallic manganese-containing ores of the Kemerovo region – Kuzbass. By thermodynamic modeling using TERRA software complex, it was determined that nickel can be completely reduced from oxide in the NiO – C system at a carbon consumption of 0.08 kg/kg NiO, and at a carbon consumption of 0.15 kg/kg NiO ‒ in the NiO – Fe2O3 – C system. It was found that cobalt reduction in the CoO – C system begins at a temperature of about 513 K at any carbon consumption. With a further increase in temperature, the reduction process depends only on consumption of the reducing agent. From the obtained thermodynamic modeling data, it follows that cobalt reduction from the cobalt-nickel concentrate begins at a temperature of about 513 K and subsequently depends slightly on temperature. The extraction of cobalt increases with the amount of reducing agent at temperatures up to 553 K, then remains constant up to 1473 K temperature. Nickel reduction takes place at a temperature above 473 K. The degree of nickel reduction slightly depends on the temperature and amount of reducing agent at consumption of the latter over 0.02 kg/kg of concentrate. Laboratory studies showed that during the melting period, nickel can be reduced from its oxide almost completely with solid carbon, since nickel has less sensitivity to oxygen than iron. Theoretical and experimental studies of steel direct alloying showed that it is advisable to use a solid phase process in reduction of nickel and cobalt. Nickel concentrate and cobalt-nickel concentrate during steel smelting in an electric furnace is advisable to be introduced into charge in the form of mixtures pelletized with a carbonaceous reducing agent.
For citations:
Rybenko I.A., Nokhrina O.I., Rozhikhina I.D., Golodova M.A. Thermodynamic modeling of cobalt and nickel reduction using hydrometallurgical enrichment concentrates for steel alloying. Izvestiya. Ferrous Metallurgy. 2024;67(4):384-390. https://doi.org/10.17073/0368-0797-2024-4-384-390
Introduction
Modern technologies must meet certain requirements, the main ones being:
– rational use of material and energy resources;
– expansion of the product range;
– improvement of product quality;
– environmental safety.
These issues are particularly relevant in the production of ferrous metals. A significant focus is on obtaining ultra-pure steel with minimal undesirable impurities through more cost-effective methods and in larger quantities, as well as altering the structure and type of alloying elements and deoxidizers used, moving towards less stringent composition requirements and corresponding cost reductions.
An analysis of current steelmaking technologies reveals that the most significant cost factors in metal production are the expenses for deoxidation and alloying of steel, as well as the technological energy required. One way to increase the efficiency of steel production is to modify the technology for obtaining alloying elements by transitioning to direct alloying of steel in the furnace or ladle using ores and concentrates [1; 2].
In the past two decades, significant attention has been paid, both in Russia [3 – 5] and abroad [6 – 8], to the search for new chemical and hydrometallurgical methods for enriching low-grade ores (including manganese ores) as part of resource conservation efforts. When enriching polymetallic manganese-containing ores from Kuzbass using the technology proposed in [9], in addition to high-quality manganese and iron concentrates, non-ferrous metal concentrates (nickel concentrate, as well as nickel-cobalt concentrate) are obtained, which can be used for direct alloying.
Technological progress in mechanical engineering, construction, chemistry, and other industries drives the demand for high-quality steels and alloys. The most in-demand types are structural, heat-resistant, acid-resistant, and stainless steels, which require non-ferrous metals in their production (with nickel being one of the primary ones).
Reserves of rich nickel ores with nickel content around 4 % are limited, leading to the use of low-grade silicate ores with nickel content up to 1.5 % [10 – 12]. Currently, there is considerable focus on finding new methods for enriching and utilizing nickel-containing ores [13 – 15]. As an alternative raw material, polymetallic manganese-containing ores and iron-manganese nodules, with nickel content up to 0.5 %, can be considered [16; 17].
Global nickel production remains steady at around 1 million tons annually. In the global economy, the demand for nickel continues to grow despite the constant rise in its price. The increasing demand for nickel is due to its wide range of applications, with the majority of produced nickel used for steel alloying.
The growing need for cobalt in various fields of science and technology, driven by the development of the aerospace industry and the production of specialty steels for oil extraction equipment, necessitates an increase in its production volume. Most cobalt produced is used to create various alloys. Cobalt is frequently used in alloys with iron, chromium, tungsten, and molybdenum. It enhances the cutting properties of high-speed steel, making it valuable in the tool industry.
Cobalt alloys possess excellent magnetic properties, corrosion resistance, wear resistance, and high thermal stability.
Adding cobalt to steel helps maintain magnetic properties at high temperatures and under vibrations, and increases resistance to demagnetization. For instance, Japanese steel containing up to 60 % Co has high coercivity and loses only 2.0 – 3.5 % of its magnetic properties under vibrations. Cobalt-based magnetic alloys are used in the production of electric motor cores, transformers, and other electrical devices [18; 19].
An analysis of the advantages and disadvantages of direct steel alloying shows that the feasibility of a particular technology is determined by technical and economic indicators such as the duration of melting and the consumption of reducing agent. The goal of the research was to maximize the replacement of silicon, the primary reducing agent for alloying elements from concentrates, with the more cost-effective carbon, and to achieve consistent recovery of nickel and cobalt from the oxides present in concentrates obtained during the hydrometallurgical enrichment of polymetallic manganese-containing ores.
Materials and methods
In the context of steel production in electric arc furnaces, reducing agents such as carbon, carbon monoxide, and silicon dissolved in molten steel can be considered at different stages of melting. To determine the conditions for the reduction of alloying elements from concentrates, thermodynamic modeling methods based on the calculation of equilibrium states in model thermodynamic systems were used [20]. Thermodynamic modeling in this study was conducted using ready-made software products (TERRA software complex) developed at Moscow State Technical University, which allows the determination of the equilibrium composition of a multicomponent, heterogeneous thermodynamic system for high-temperature conditions based on the principle of maximum entropy.
An elementary system is formed by specifying the quantity of its constituent components and temperature. Condensed solution compositions are formed if necessary. For the selected two thermodynamic parameters, multi-variant calculations of equilibrium compositions are carried out depending on the thermodynamic parameters or the consumption of raw materials.
The set of substances that can form given the specified elemental composition of the mixture was determined through numerical modeling for the chosen temperature range and various thermodynamic states. From the complete list of possible substances, only those whose concentrations exceeded 10\(^‒\)4 mol/kg of the mixture were selected. Calculations were conducted in the temperature range from 573 to 1873 K, corresponding to steelmaking temperatures.
Thermodynamic modeling of nickel, cobalt, and combined nickel and cobalt reduction was performed for pure systems and concentrates obtained from the enrichment of polymetallic manganese raw materials: nickel concentrate (45.0 % Ni; 2.3 % Mn; 1.4 % Fe; 0.5 % Co; 0.1 % Cu; less then 0.015 % P; traces of SiO2 and 2,82 % loss on ignition); cobalt-nickel concentrate (76.8 % CoO; 11.9 % NiO; 4.9 % Fe2O3 ; 1.2 % Mn2O3 ; 0.2 % SiO2 ; 1.2 % CaCl2 ; 3.8 % loss on ignition) and coke from EVRAZ United West Siberian Metallurgical Plant (A\(^d\) = 13.6 %; V\(^{daf}\) = 2 %; W\(^p\) = 2 %; 51.1 % SiO2 ; 23.3 % Al2O3 ; 0.16 % MnO2 ; 1.58 % MgO; 1.2 % CaO; 17.46 % Fe2O3 ; 0.5 % P2O5 ; 1.2 % K2O; 0.2 % Na2O; 74.4 % СО2 ).
During laboratory studies, an optimal method for introducing nickel oxide into an electric arc furnace was experimentally determined and tested. Steel was smelted in a 10 kg capacity laboratory electric arc furnace. Pellets with a diameter of 20 – 30 mm were produced from nickel concentrate obtained from the enrichment of polymetallic manganese ores (with a fraction size less than 0.5 mm) and coke fines. The pellets were charged into the furnace in two ways: I – into the charge; II – during the reduction period, onto the metal bath before slag formation.
The number of pellets was calculated to achieve 1 % nickel content in the steel. Experimental heats were conducted using the classical two-slag technology. Scrap metal with the following composition, wt. %, was used: 0.275 C; 0.267 Si; 0.423 Mn; 0.175 Cr; 0.1 Ni; 0.027 S; 0.028 P; the remainder Fe. The mass of charge materials for experimental heat is provided in the Table. After smelting, samples of metal and slag were taken for chemical analysis. The metal and slag were tapped into a ladle. The resulting ingot was cut into three equal parts along its height for metal analysis.
Initial charge materials for experimental heats
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Results and discussion
Nickel reduction from nickel concentrate oxides
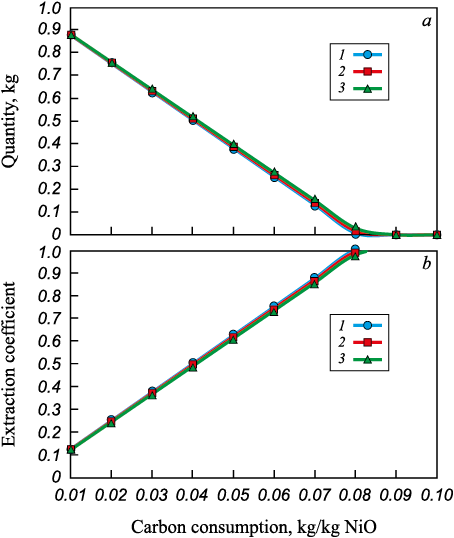
The calculation of possible compositions resulting from nickel reduction processes in the thermodynamic system consisting of NiO – C was performed by varying the carbon content in the system, allowing the evaluation of the concentration limits for reduction processes. The model system NiO – C was formed by specifying the initial composition of the mixture: 1 kg of NiO and carbon in amounts ranging from 0.01 to 0.1 kg/kg of oxide. The studies were conducted in the temperature range from 1073 to 1873 K. The dependence of nickel recovery coefficients from the oxide on the amount of carbon at temperatures of 1073, 1473, and 1873 K are presented in Fig. 1. Thermodynamic calculations showed that maximum nickel reduction is achieved at a carbon consumption of 0.08 kg/kg NiO. Therefore, complete nickel reduction can be achieved during solid-phase reduction before the appearance of a liquid phase.
Fig. 1. Dependences of nickel reduction parameters on the amount of |
The results of equilibrium state calculations for the NiO – Fe2O3 – C system within a carbon range of 0.01 – 0.50 kg/kg NiO and an initial amount of NiO (1 kg) and Fe2O3 (2 kg) are presented in Fig. 2. The significant condensed phase consists of Ni, NiO, C, FeO, and Fe2O3 . The gas phase is represented by CO and CO2 oxides.
Fig. 2. Dependence of slag phase composition on carbon flow rate |
The slag phase of the considered system is represented by FeO, Fe2O3 , and NiO oxides. When the amount of reducing agent exceeds 0.2 kg/kg NiO, the slag phase consists only of FeO. Complete nickel reduction from oxide occurs at a carbon content of 0.15 kg/kg NiO in the NiO – Fe2О3 – C system.
The results of thermodynamic modeling demonstrated that nickel can be fully reduced from its oxide in both the NiO – C system and the NiO – Fe2O3 – C system at metallurgical process temperatures.
During laboratory experiments, the optimal method for introducing nickel oxide into the electric arc furnace was experimentally determined. In heats 1 to 3, pellets made from nickel concentrate were used, while in heats 4 to 6, a mixture of nickel concentrate and coke was used (see Table).
The results of experimental steel alloying heats using nickel-containing pellets showed that nickel recovery from the concentrate was 92 – 95 % (option I) and 75 – 78 % (option II).
The decrease in nickel recovery when it was added at the beginning of the reduction period is likely due to partial evaporation; as nickel is reduced in the arc zone, it may partially evaporate due to its relatively low boiling point может частично испаряться, так как имеет относительно низкую температуру кипения.
Under steelmaking conditions in an electric arc furnace, within the temperature range of 1173 – 1873 K, nickel can be almost completely reduced from its oxide by solid carbon during the melting period, as nickel has a lower affinity for oxygen than iron.
Cobalt reduction in elementary systems
The study of cobalt oxide dissociation revealed that the highest oxide, Co2O3 , is absent in the system at temperatures above 473 K. The cobalt oxide stable at room temperature is the complex oxide Co3O4 , which has a spinel structure where one set of lattice sites is occupied by Co\(^{2+}\) ions and the other by Co\(^{3+}\) ions; it decomposes to form CoO at temperatures above 1173 K. The results of thermodynamic calculations are shown in Fig. 3. The dependencies indicate that Co3O4 remains stable up to 1173 K. In the temperature range of 1173 to 1223 K, the amount of Co3O4 decreases to zero, CoO appears at 1173 K, and its amount becomes maximal and stable to temperature changes at 1223 K.
Fig. 3. Thermal dissociation of cobalt oxides: |
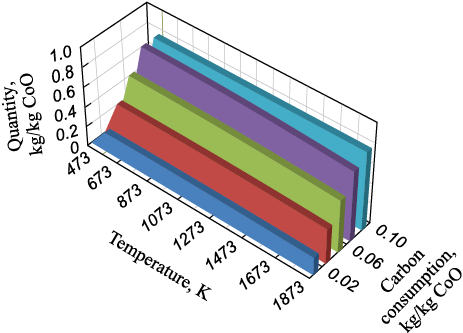
In modeling cobalt reduction, the CoO – C system was considered. Calculations were performed in the temperature range of 273 to 1273 K, with reducing agent (carbon) consumption ranging from 0.02 to 0.10 kg/kg CoO. Thermodynamic modeling showed that cobalt reduction begins at approximately 513 K, regardless of carbon consumption. As the temperature increases, the reduction process depends solely on the amount of reducing agent (Fig. 4). Cobalt is fully reduced from its oxide at a carbon consumption of 0.1 kg/kg CoO at 1273 K.
Fig. 4. Dependence of the amount of reduced cobalt on temperature |
Thermodynamic calculations of Co reduction in the Co – O – Si and Co – O – Al systems in the temperature range of 273 to 1273 K showed that cobalt is fully reduced at a silicon consumption of 0.1 kg/kg Co or at an aluminum consumption of 0.24 kg/kg CoO at 1273 K.
Nickel and cobalt reduction from cobalt-nickel concentrate
The reduction of cobalt and nickel from the oxides in the concentrate using coke was studied in the temperature range of 273 to 2073 K. Coke consumption varied from 0.02 to 0.10 kg/kg of concentrate.
The significant reduction products included:
– cobalt, nickel, cobalt oxides, nickel oxides, iron, manganese, calcium and magnesium silicates, and aluminates (condensed phase);
– water vapor, CO and CO2 oxides, metal chlorides (gas phase); chlorides were detected at temperatures above 1513 K.
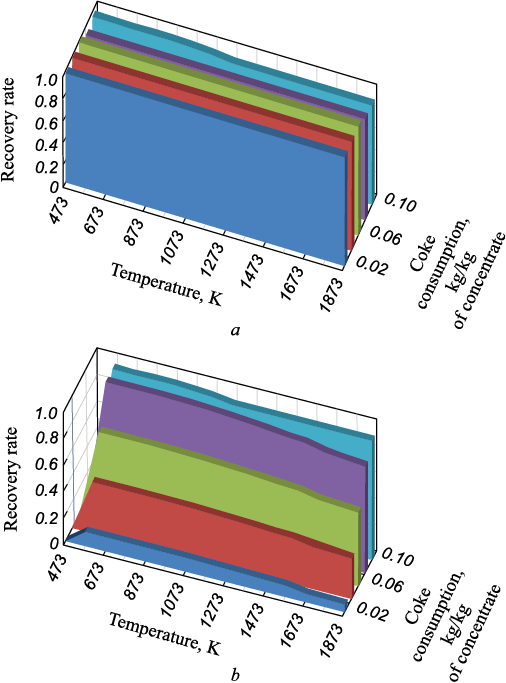
The dependencies of cobalt and nickel recovery coefficients from the cobalt-nickel concentrate on temperature and carbon consumption are shown in Fig. 5.
Fig. 5. Dependences of extraction coefficients of nickel (a) |
The thermodynamic data indicate that cobalt reduction begins at approximately 513 K and is minimally affected by further temperature increases. The degree of cobalt recovery increases with the amount of reducing agent up to 553 K, after which it remains constant up to 1473 K. With further temperature increases, cobalt recovery decreases. When calculating the cobalt recovery coefficient, only cobalt present in the condensed phase was considered. The gas phase contained CoCl2 , and its amount increased with rising temperature.
Nickel reduction occurs at temperatures above 473 K. The degree of nickel reduction is slightly dependent on temperature and amount of reducing agent when the latter exceeds 0.02 kg/kg of concentrate.
The study results showed that during the reduction of nickel and cobalt from concentrates, complete reduction of these elements is achieved at solid-phase reduction temperatures in the range of 573 to 1073 K. The data obtained are consistent with the results of previous studies [18; 19].
Conclusions
The results of theoretical and experimental studies suggest that solid-phase reduction is advisable for nickel and cobalt reduction. Nickel can be fully reduced from nickel concentrate by carbon at 1073 K, while cobalt and nickel reduction from cobalt-nickel concentrate begins at approximately 513 K and is minimally affected by further temperature increases. Therefore, it is advisable to introduce nickel and cobalt-nickel concentrates into the furnace charge during steelmaking in electric arc furnaces as mixtures pelletized with a carbonaceous reducing agent.
References
1. Nokhrina O. I., Rozhikhina I.D., Dmitrienko V.I. Platonov M.A. Alloying and Modification of Steel using Natural and Man-Made Materials. Tomsk: Izd-vo Tomskogo politekhnicheskogo universiteta; 2013:320. (In Russ.).
2. Nosov Yu.N., Kamshukov V.P., Sokolov V.V., etc. Direct alloying of steel with manganese agglomerate in a ladle at converter tapping. Stal’. 2004;(5):35–36. (In Russ.).
3. Nokhrina O.I., Rozhikhina I.D., Edil’baev A.I., Edil’baev B.A. Manganese ores of the Kemerovo region – Kuzbass and methods of their enrichment. Izvestiya. Ferrous Metallurgy. 2020;63(5):344–350. (In Russ.). https://doi.org/10.17073/0368-0797-2020-5-344-350
4. Chernobrovin V.P., Mizin V.G., Sirina T.P., Dashevskii V.Ya. Complex Processing of Carbonate Manganese Raw Materials: Chemistry and Technology. Chelyabinsk: SUSU; 2009:294. (In Russ.).
5. Kurkov A.V., Mamoshin M.Yu., Rogozhin A.A. Breakthrough Hydrometallurgical Processes for the Sustainable Development of Mineral Processing Technologies. Moscow: VIMS; 2019:106. (In Russ.).
6. Pan M.C., Liu X.L., Zou R., Huang J., Han J.C. Study of heat treatment technology on medium-carbon-low-alloy-steel large hammer formation of gradient performance. Advanced Materials Research. 2014;881-883:1288–1292. http://dx.doi.org/10.4028/www.scientific.net/AMR.881-883.1288
7. Sun D., Li M.L., Li C.H., Cul R., Zheng X.Y. A green enriching process of Mn from low grade ore of manganese carbonate. Applied Mechanics and Materials. 2014;644–650: 5427–5430. http://dx.doi.org/10.4028/www.scientific.net/AMM.644-650.5427
8. Ayala J., Fernandez B. Recovery of manganese from silicomanganese slag by means of a hydrometallurgical process. Hydrometallurgy. 2015;158:68–73. https://doi.org/10.1016/j.hydromet.2015.10.007
9. Nokhrina O.I., Rozhikhina I.D., Rybenko I.A., Golodova M.A., Izrail’skii A.O. Hydrometallurgical enrichment of polymetallic and ferromanganese ore. Izvestiya. Ferrous Metallurgy. 2021;64(4):273–281. (In Russ.). https://doi.org/10.17073/0368-0797-2021-4-273-281
10. Pat. 7125436 USA, IPC7 from 22 at 15/00, from 22 at 11/08. The method of autoclave leaching of nickel ores. Run L., Maurt T. No. 10/907324. Published on 10.24.2006. NPC 75/724.
11. Wang K., Li J, McDonald R.G., Browner R.E. The effect of iron precipitation upon nickel losses from synthetic atmospheric nickel laterite leach solutions: Statistical analysis and modeling. Hydrometallurgy. 2011;109(1-2):140–152. http://dx.doi.org/10.1016/j.hydromet.2011.06.009
12. Anjum F., Shahid M., Akcil A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy. 2012;117–118:1–12. http://dx.doi.org/10.1016/j.hydromet.2012.01.007
13. Giannopoulou I., Panias D. Differential precipitation of copper and nickel from acidic polymetallic aqueous solutions. Hydrometallurgy. 2008;90(2–4):137–146. https://doi.org/10.1016/j.hydromet.2007.10.004
14. 1731623 EVP, IPC from 22 to 23/00. Method for teaching and extraction of nickel and cobalt. H. Yakushiji, S. Ito, K. Jura, M. Shimamori. Published on 12.13.2006.
15. Coto O., Galizia F., Hernandez I., Marrero J., Donati E. Cobalt and nickel recoveries from laterite tailings by organic and inorganic bioacids. Hydrometallurgy. 2008;94(1–4):18–22. http://dx.doi.org/10.4028/www.scientific.net/AMR.20-21.107
16. Ntuli F., Lewis A.E. Kinetic modelling of nickel powder precipitation by high-pressure hydrogen reduction. Chemical Engineering Science. 2009;64(9):2202–2215. http://dx.doi.org/10.1016/j.ces.2009.01.026
17. Proshunin I.E., Nokhrina O.I. Complex extraction of valuable components from ferromanganese nodules. Izvestiya. Ferrous Metallurgy. 2009;52(8):17–19. (In Russ.).
18. Kozlov P.A. Investigation of the effect of alloying on the composition of heat- resistant 9 % chromium steels for parts of thermal power equipment: Extended Abstract of Cand. Sci. Diss. 05.16.02. Moscow; 2011:25. (In Russ.).
19. Kniss V.A. Physico-chemical bases and technology of reduction melting of cobalt oxide in electric furnaces of direct current. Extended Abstract of Cand. Sci. Diss. 05.16.02. Yekaterinburg; 2008:31. (In Russ.).
20. Trusov B.G. TERRA software system for modeling phase and chemical equilibria at high temperatures. In: III Int. Symp.”Combustion and Plasma Chemistry”. August 24–26, 2005, Almaty, Kazakhstan. Almaty: Kazak universiteti; 2005:52–57. (In Russ.).
About the Authors
I. A. RybenkoRussian Federation
Inna A. Rybenko, Dr. Sci. (Eng.), Prof., Head of the Chair of Applied Information Technologies and Programming
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
O. I. Nokhrina
Russian Federation
Ol’ga I. Nokhrina, Dr. Sci. (Eng.), Prof. of the Chair of Ferrous Metallurgy and Chemical Technology
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
I. D. Rozhikhina
Russian Federation
Irina D. Rozhikhina, Dr. Sci. (Eng.), Prof. of the Chair of Ferrous Metallurgy and Chemical Technology
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
M. A. Golodova
Russian Federation
Marina A. Golodova, Cand. Sci. (Eng.), Assist. Prof. of the Chair of Architecture
42 Kirova Str., Novokuznetsk, Kemerovo Region – Kuzbass 654007, Russian Federation
Review
For citations:
Rybenko I.A., Nokhrina O.I., Rozhikhina I.D., Golodova M.A. Thermodynamic modeling of cobalt and nickel reduction using hydrometallurgical enrichment concentrates for steel alloying. Izvestiya. Ferrous Metallurgy. 2024;67(4):384-390. https://doi.org/10.17073/0368-0797-2024-4-384-390
JATS XML