Scroll to:
Physical and chemical processes during nitriding of chromium ferrosilicon by filtration combustion
https://doi.org/10.17073/0368-0797-2024-3-360-365
Abstract
In this paper, the nitriding of chromium ferrosilicon is carried out in the combustion mode under the condition of natural nitrogen filtration. The authors studied the effect of the key parameters (pressure of gaseous nitrogen, diameter and dispersity of starting samples) on the maximum temperature and combustion of the starting powder mixture based on chromium ferrosilicon. The combustion synthesis of chromium ferrosilicon proceeds steadily in the stationary mode with formation of a macrohomogeneous nitrided composition which, according to the results of X-ray phase analysis, contains two nitride phases - chromium nitride and silicon nitride. Interaction of the initial powder with gaseous nitrogen in the filtration combustion mode proceeds by the following probable chemical reaction: 3CrSi2 + 3Si + 3FeSi2 + 11.5N2 = 3CrN + 5Si3N4 + 3Fe. Increasing the diameter of the starting samples slightly affects the amount of absorbed nitrogen and slows the propagation of the combustion wave front. An increase in the pressure of gaseous nitrogen increases the amount of absorbed nitrogen and the combustion rate. Increasing the dispersity of the starting powder increases the amount of absorbed nitrogen and the combustion rate. It was found that the combustion reaction is not possible with a dense initial sample. The maximum combustion temperature, depending on the nitriding conditions, varies between 2400 and 2650 °C and increases with increasing gaseous nitrogen pressure, diameter of the initial samples and dispersion of chromium ferrosilicon powder. It is possible to realize nitriding of chrome ferrosilicon in the combustion mode at the pressure of gaseous nitrogen not less than 3 MPa, diameter of initial samples not less than 3.5 cm and size of initial particles not more than 100 μm. Optimal parameters of nitriding are gaseous nitrogen pressure of 5 MPa, diameter of samples 5 cm, size of initial particles less than 100 μm and bulk density of samples (2.23 g/cm3).
Keywords
For citations:
Bolgaru K.A., Reger A.A., Vereshchagin V.I., Akulinkin A.A. Physical and chemical processes during nitriding of chromium ferrosilicon by filtration combustion. Izvestiya. Ferrous Metallurgy. 2024;67(3):360-365. https://doi.org/10.17073/0368-0797-2024-3-360-365
Introduction
The method of self-propagating high-temperature synthesis (SHS) is based on highly exothermic reactions that occur in the form of a combustion wave in a self-propagating mode. The SHS method has undeniable advantages: energy efficiency, short synthesis time, environmental friendliness, and simplicity of equipment [1 – 3].
Currently, a large number of materials, particularly nitrides, have been obtained by the filtration SHS method in a nitrogen environment [4 – 6]. Several nitride materials possess unique physicochemical properties [7 – 9]. They can be used in the production of gas turbine components [10], heat-dissipating radiators [11], cutting tools [12; 13], photocatalysts [14], semiconductors [15], and so on.
The most promising application in the filtration SHS method is the use of accessible and relatively inexpensive ferroalloys. Using ferroalloys in SHS processes can produce nitride material at a relatively low cost without losing product quality [16; 17]. Iron, which is part of the ferroalloys, has a catalytic effect on the nitriding process of other elements included in the initial mixture [18]. Thus, iron increases the intensity and depth of nitriding of the initial material. There is a considerable amount of work dedicated to the filtration combustion of simple ferroalloys. The patterns of nitriding of ferrosilicon [19 – 21], as well as the filtration combustion of ferrochrome and ferrovanadium [22], have been thoroughly studied. The study [23] investigated the combustion of industrial ferrotitanium in nitrogen. The monograph [24] described the nitriding of ferroboron and ferroniobium in a combustion mode.
However, the use of complex ferroalloys in filtration combustion processes is interesting and little studied. Complex ferroalloys are alloys of iron with two or more elements. At present, SHS combustion of ferro-silicoaluminium [25] and ferro-aluminum-silicon-zirconium [2] has been studied.
The aim of this work was to study the combustion processes of chromium ferrosilicon in a self-propagating mode under conditions of natural nitrogen filtration to obtain a nitride-containing composite material based on chromium nitride and silicon nitride.
Materials and methods
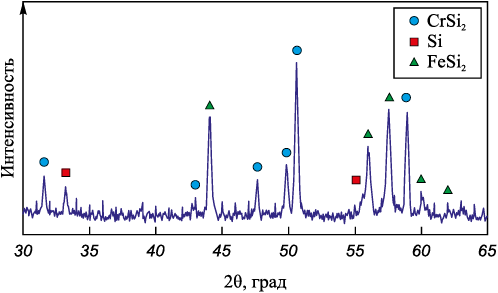
Chromium ferrosilicon (CFS) was used as the starting material. X-ray phase analysis showed that this ferroalloy is multiphase and contains CrSi2 , Si, and FeSi2 (Fig. 1). According to the chemical analysis, the composition of CFS is as follows (wt. %): 49.4 Si, 29.7 Cr, 20.7 Fe, and the rest are oxides. For nitriding in the self-propagating mode, the initial CFS was ground in a ball mill and dried in a vacuum drying oven at a temperature of 150 °C for 3 h.
Fig. 1. X-ray diffraction pattern of chromium ferrosilicon |
Nitriding of the initial CFS was carried out in a constant pressure setup with a volume of 3 liters. For the synthesis, the initial powder mixture was placed in a gas-permeable container mounted on a non-conductive stand. An igniting composition was poured over the initial charge. A coil was connected to the igniting composition to conduct an electric pulse from a transformer. After the electric pulse was applied, the combustion reaction of the igniting composition was initiated. Then, the heat released as a result of the combustion of the igniting composition initiated the combustion reaction of the initial CFS powder. After the combustion wave front passed and complete cooling occurred, the unreacted nitrogen was vented, and the nitrided samples were removed for further physicochemical studies.
The phase composition was studied using a Shimadzu XRD-6000 diffractometer. The oxygen and nitrogen contents were determined using a LEKO-ONH 836 instrument. The maximum combustion temperature was measured using the thermocouple method with tungsten-rhenium thermocouples (WRe5-WRe20) on an LA20USB.
Results and discussion
Combustion of chromium ferrosilicon proceeds in a stationary mode. The nitrided samples obtained based on CFS are macrohomogeneous. An image of the nitrided CFS is shown in Fig. 2.
Fig. 2. Sample of nitrided chromium ferrosilicon |
The probable chemical reactions of the interaction of the initial charge based on CFS with nitrogen are given below:
| 3CrSi2 + 5.5N2 = 3CrN + 2Si3N4 ; | (1) |
| 3Si + 2N2 = Si3N4 ; | (2) |
| 3FeSi2 + 4N2 = 2Si3N4 + 3Fe. | (3) |
The overall chemical reaction equation is as follows:
| 3CrSi2 + 3Si + 3FeSi2 + 11.5N2 = 3CrN + 5Si3N4 + 3Fe. | (4) |
Reaction (4) corresponds to the complete nitriding of the initial CFS (with a conversion degree of 1). Due to the rapid processes of SHS, the initial charge is in the reaction zone for a relatively short time and does not fully react with nitrogen. It is theoretically calculated that the maximum amount of absorbed nitrogen by chromium ferrosilicon is 28.99 %.
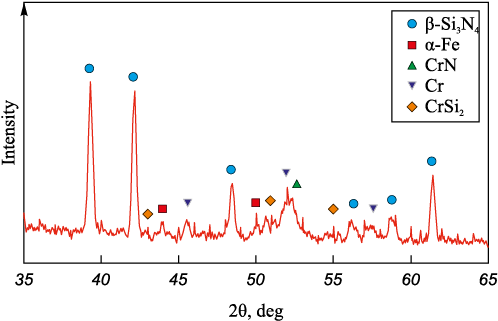
The nitrided CFS product is a multiphase material containing β-Si3N4 , α-Fe, CrN, Cr and CrSi2 . The presence of Cr and CrSi2 indicates the incomplete nitriding reaction of the initial powder (Fig. 3).
Fig. 3. X-ray diffraction pattern of nitrided chromium ferrosilicon |
Parameters such as the pressure of the gaseous reactant, sample diameter, particle size, and density of the initial material significantly influence the maximum temperature, process, and feasibility of filtration combustion in a self-propagating mode.
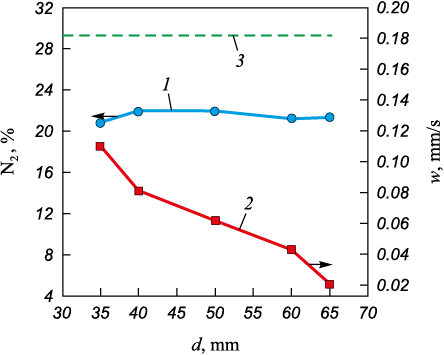
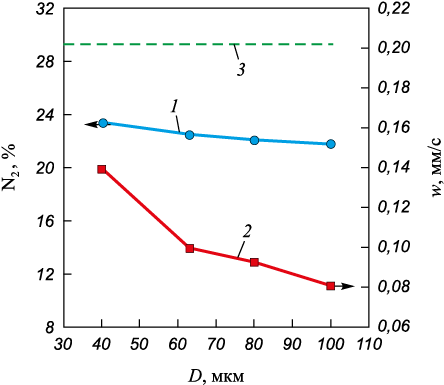
Fig. 4 shows the dependence of the amount of absorbed nitrogen and the combustion rate on the diameter of the initial samples. The influence of the diameter was studied in the range of 35 to 65 mm. Combustion of CFS can be initiated with initial sample diameters of at least 35 mm. Increasing the diameter slightly affects the amount of absorbed nitrogen and leads to a decrease in the combustion rate from 0.11 to 0.021 mm/s. This slight change in the amount of absorbed nitrogen is due to the increased difficulty of nitrogen filtration reaching the reaction zone as the diameter increases. At the same time, due to the slowdown in the combustion wave front, the residence time of the initial CFS particles in the reaction zone increases. The slowdown of the combustion wave front is related to the increased volume of the powder mixture, which requires a large amount of heat for heating. When changing the diameter, the maximum amount of absorbed nitrogen was about 22 %, which is 6.99 % less than the theoretically calculated amount of nitrogen absorption. As the diameter of the initial powder mixture increases, the maximum combustion temperature of CFS changes from 2400 to 2650 °С.
Fig. 4. Dependence of the content of absorbed nitrogen (1) |
In a laboratory setup with a volume of 3 liters, it is preferable to implement combustion of samples with a diameter of 50 mm.
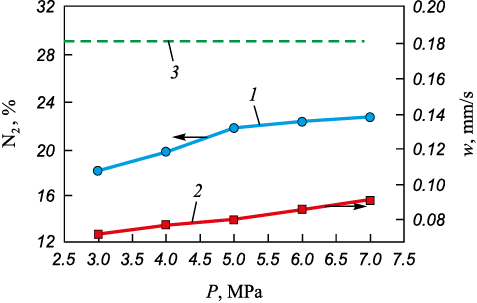
Increasing the pressure accelerates the filtration of gaseous nitrogen and, accordingly, increases the concentration of the reagent gas in the chemical reaction zone. Combustion of CFS at a nitrogen pressure of less than 3 MPa could not be achieved. Increasing the pressure of gaseous nitrogen leads to an increase in the amount of absorbed nitrogen from 18.3 to 22.8 % and the combustion rate from 0.073 to 0.092 mm/s. In the nitrogen pressure range from 5 to 7 MPa, the change in the amount of absorbed nitrogen becomes less pronounced. Increasing the nitrogen pressure above 7 MPa is not advisable because, at 5 MPa, the influence of pressure on the combustion process becomes insignificant (Fig. 5). When implementing CFS combustion at a pressure of 7 MPa, the sample contains 6.29 % less nitrogen than the theoretically calculated value. As the pressure of gaseous nitrogen increases from 3 to 7 MPa, the maximum combustion temperature rises from 2350 to 2600 °С.
Fig. 5. Dependence of the content of absorbed nitrogen (1) |
Combustion of CFS with particle sizes greater than 100 µm and without fine fractions (less than 63 µm) could not be achieved. As the particle size of the initial material decreases, the amount of absorbed nitrogen increases from 21.9 to 23.5 % and the combustion rate from 0.081 to 0.140 mm/s. The increase in the dispersity of the initial material leads to an increase in the specific surface area capable of reacting (Fig. 6). The reduction in particle size of CFS results in an increase in the maximum temperature from 2400 to 2490 °С.
Fig. 6. Dependence of the content of absorbed nitrogen (1) |
The increase in the density of the initial powder mixture was achieved by pressing the initial powder into tablets with a diameter and height of 40 mm in molds. Combustion of the pressed powder, which retains the shape of the tablet (ρ = 2.52 g/cm3), could not be achieved. Therefore, only samples with a bulk density (2.23 g/cm3) were used.
Conclusions
The combustion of chromium ferrosilicon proceeds in a stationary mode, resulting in homogeneous nitrided samples without melt droplets or cracks.
Increasing the diameter leads to a decrease in the combustion rate from 0.11 to 0.021 mm/s and slightly affects the amount of absorbed nitrogen. Increasing the nitrogen pressure results in an increase in the amount of absorbed nitrogen (18.3 – 22.8 %) and the combustion rate (0.073 – 0.092 mm/s) of chromium ferrosilicon. Reducing the dispersity of the initial material allows for an increase in the amount of absorbed nitrogen from 21.9 to 23.5 % and the combustion rate from 0.081 to 0.140 mm/s. A slight increase in the density of the initial powder prevents achieving the combustion reaction of chromium ferrosilicon in a nitrogen environment.
The maximum combustion temperature increases with a rise in gaseous nitrogen pressure from 3 to 7 MPa (from 2350 to 2600 °C), the diameter of the initial samples from 35 to 65 mm (from 2400 to 2650 °C), and a decrease in the particle size of the initial charge from less than 100 to less than 40 µm (from 2400 to 2490 °С).
Stable combustion in the self-propagating mode of chromium ferrosilicon powder samples is possible at a nitrogen pressure of at least 3 MPa, a sample diameter of at least 3.5 cm, a particle size of less than 100 µm with the presence of fine fractions (less than 63 µm), and a sample density not exceeding 2.23 g/cm3. It is optimal to carry out nitriding of the initial chromium ferrosilicon under conditions of natural nitrogen filtration at a pressure of 5 MPa, a sample diameter of 5 cm, a particle size of the initial material less than 100 µm, and a bulk sample density of 2.23 g/cm3.
The product of chromium ferrosilicon nitriding contains β-Si3N4 , CrN, α-Fe, Cr and CrSi2 . The presence Cr and CrSi2 indicates the incomplete nitriding reaction of the initial chromium ferrosilicon. The nitrogen saturation is 21.9 %, which is 7.09 % less than the theoretically calculated maximum amount of absorbed nitrogen.
References
1. Chukhlomina L.N., Vitushkina O.G. SHS nitriding of ferrosilicon with ilmenite. ChemChemTech. 2011;54(5):105–108. (In Russ.).
2. Bolgaru K.A., Vereshchagin V.I., Reger A.A. Synthesis of composite of silicon nitride, aluminium nitride, zirconium nitride from complex ferroalloy – ferro-aluminium-silicon-zirconium by nitriding in combustion mode. ChemChemTech. 2021;64(7):68–74. (In Russ.). https://doi.org/10.6060/ivkkt.20216407.6362
3. Amosov A.P., Borovinskaya I.P., Merzhanov A.G. Powder Technology of Self-Propagating High-Temperature Synthesis of Materials. Moscow: Mashinostroenie. 2007:567.
4. Won H.I., Won C.W., Nersisyan H.H., Yoon K.S. Salt-assisted combustion synthesis of silicon nitride with high α-phase content. Journal of Alloys and Compounds. 2010;496(1-2): 656–659. https://doi.org/10.1016/j.jallcom.2010.02.157
5. Yeh C.L., Liu E.W. Combustion synthesis of chromium nitrides by SHS of Cr powder compacts under nitrogen pressures. Journal of Alloys and Compounds. 2006;426(1-2): 131–135. https://doi.org/10.1016/j.jallcom.2006.01.082
6. Yeh C.L., Chuang H.C. Experimental studies on self-propagating combustion synthesis of niobium nitride. Ceramics International. 2004;30(5):733–743. https://doi.org/10.1016/j.ceramint.2003.10.001
7. Zhang J., Ge Y., Tian Z., Sun S., Cui W., Liu G., Chen K. Combustion synthesis of α-Si3N4 with green additives. Ceramics International. 2019;45(5):6594–6596. https://doi.org/10.1016/j.ceramint.2018.12.147
8. Zhang Y., He X., Han J., Du S. Combustion synthesis of hexagonal boron–nitride-based ceramics. Journal of Materials Processing Technology. 2001;116(2-3):161–164. https://doi.org/10.1016/S0924-0136(01)01023-8
9. Chen Y.X., Li J.T., Du J.S. Cost effective combustion synthesis of silicon nitride. Materials Research Bulletin. 2008;43(6):1598–1606. https://doi.org/10.1016/j.materresbull.2007.06.051
10. Won H.I., Won C.W., Nersisyan H.H., Yoon K.S. Salt-assisted combustion synthesis of silicon nitride with high α-phase content. Journal of Alloys and Compounds. 2010;496(1–2): 656–659. https://doi.org/10.1016/j.jallcom.2010.02.157
11. Juang R.C., Chen C.C. Combustion synthesis of hexagonal aluminum nitride crystal by aluminum carbides. Materials Science and Engineering: A. 2007;458(1–2):210–215. https://doi.org/10.1016/j.msea.2007.01.004
12. Fang S. Morphological study of a cubic boron nitride (CBN) cutting tool and characterization of its wear scenarios in abrasive machining process. Ceramics International. 2020;46(11B):19491–19498. https://doi.org/10.1016/j.ceramint.2020.04.302
13. Sugihara T., Nishimoto Y., Enomoto T. Development of a novel cubic boron nitride cutting tool with a textured flank face for high-speed machining of Inconel 718. Precision Engineering. 2017;48:75–82. https://doi.org/10.1016/j.precisioneng.2016.11.007
14. Akulinkin A., Bolgaru K., Reger A. Facile synthesis of porous g-C3N4/β-SiAlON material with visible light photocatalytic activity. Materials Letters. 2021;305:130788. https://doi.org/10.1016/j.matlet.2021.130788
15. Hua Q., Ma B., Hu W. Aluminum, gallium, and indium nitrides. Encyclopedia of Materials: Technical Ceramics and Glasses. 2021;3:74–83. https://doi.org/10.1016/B978-0-12-803581-8.12065-X
16. Ziatdinov M.Kh. From the history of nitrided ferroalloys. Izvestiya. Ferrous Metallurgy. 2020;63(10):773–781. (In Russ.). https://doi.org/10.17073/0368-0797-2020-10-773-781
17. Manashev I.R., Gavrilova T.O., Shatokhin I.M., Ziatdinov M.Kh., Leont’ev L.I. Utilization of dispersed waste of ferroalloy production on the basis of metallurgical SHS-process. Izvestiya. Ferrous Metallurgy. 2020;63(8):591–599. (In Russ.). https://doi.org/10.17073/0368-0797-2020-8-591-599
18. Zhang M., Chen Z., Huang J., Wang S., Xiong Q., Feng Z., Liu Q., Sun Z., Li X. In situ nitriding reaction formation of β-Sialon with fibers using transition metal catalysts. Ceramics International. 2019;45(17A):21923–21930. https://doi.org/10.1016/j.ceramint.2019.07.204
19. Chukhlomina L.N., Maksimov Yu.M., Kitler V.D., Vitushkina O.G. Mechanism and features of nitriding of ferrosilicon in the combustion regime. Combustion, Explosion, Shock Waves. 2006;42(3):309–316. https://doi.org/10.1007/s10573-006-0056-0
20. Ma C., Li Y., Chen J., Zhu S., Li B. Cost-effective manufacture and synthesis mechanism of ferrosilicon nitride porous ceramic with interlocking structure. Ceramics International. 2021;47(4):5265–5272. https://doi.org/10.1016/j.ceramint.2020.10.107
21. Ziatdinov M.Kh., Shatokhin I.M. Self-propagating high-temperature synthesis of ferrosilicon nitride. Steel in Translation. 2008;38(1):39–44. https://doi.org/10.3103/S0967091208010130
22. Ziatdinov M.Kh., Shatokhin I.M., Leont’ev L.I. SHS technology of composition ferroalloys. Part I. Metallurgical SHS process. Synthesis of ferrovanadium and ferrochromium nitrides. Izvestiya. Ferrous Metallurgy. 2018;61(5):339–347. (In Russ.). https://doi.org/10.17073/0368-0797-2018-5-339-347
23. Glazunov A.A., Maksimov Yu.M., Chukhlomina L.N., Braverman B.Sh., Avramchik A. N. Combustion of ferrotitanium in nitrogen. Combustion, Explosion, and Shock Waves. 2020;56(2):137–141. https://doi.org/10.1134/S0010508220020033
24. Chukhlomina L.N., Maksimov Yu.M., Vereshchagin V.I. Self-Propagating High–Temperature Synthesis of Composites Nitride-Based Materials. Novosibirsk: Nayka; 2012:260. (In Russ.).
25. Bolgaru K., Reger A., Verechchagin V., Akulinkin A. Combustion synthesis of porous ceramic β-Si3N4-based composites with the use of ferroalloys. Ceramics International. 2021;47(24):34765–4773. https://doi.org/10.1016/j.ceramint.2021.09.015
About the Authors
K. A. BolgaruRussian Federation
Konstantin A. Bolgaru, Cand. Sci. (Eng.), Senior Researcher
10/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
A. A. Reger
Russian Federation
Anton A. Reger, Junior Researcher
10/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
V. I. Vereshchagin
Russian Federation
Vladimir I. Vereshchagin, Dr. Sci. (Eng.), Prof.
30 Lenina Ave., Tomsk 634050, Russian Federation
A. A. Akulinkin
Russian Federation
Aleksandr A. Akulinkin, Junior Researcher
10/4 Akademicheskii Ave., Tomsk 634055, Russian Federation
Review
For citations:
Bolgaru K.A., Reger A.A., Vereshchagin V.I., Akulinkin A.A. Physical and chemical processes during nitriding of chromium ferrosilicon by filtration combustion. Izvestiya. Ferrous Metallurgy. 2024;67(3):360-365. https://doi.org/10.17073/0368-0797-2024-3-360-365