Scroll to:
Nitrogen diffusion along the layer boundaries after nitriding of multilayer materials
https://doi.org/10.17073/0368-0797-2024-3-318-324
Abstract
Diffusion processes play a key role in formation of the structures of new materials and technological processes of strengthening heat treatments, since diffusion is the reason for redistribution of substances in solids. An urgent task is to develop technologically advanced and effective methods for strengthening materials in order to improve their performance properties. There is an increasing need to improve chemical heat treatment methods, which directly affects the wear resistance of working surfaces, and, consequently, the product service life. Near-surface volumes experience increased loads, so the formation of high-strength layers becomes an important task. Quite a few methods of surface hardening are known, among which carburization, nitriding, nitrocarburization and others are widely used. The most interesting is nitriding, since it increases hardness, strength, fatigue limit, and heat resistance. However, despite the proper advantages, nitriding has a number of disadvantages, including the holding duration and small thickness of diffusion layers. The solution is related to intensification of the technological process by increasing the nitriding temperature, activating the nitriding media or directly the parts surface. All these solutions are aimed at accelerating diffusion processes, both in grain volume and along grain boundaries, the velocity along which is many times higher than the velocity of volumetric diffusion. It may be effective to use a new type of structural metal materials with a multilayer structure of hundreds of layers, with thicknesses in the micron and submicron ranges separated by large angular boundaries. The results of metallographic studies showed the effect of the steel layers interchange in the multilayer metal material on diffusion depth after chemical heat treatment. The authors proposed an accelerate diffusion model of diffusible element along the layer boundaries.
Keywords
For citations:
Polikevich K.B., Petelin A.L., Plokhikh A.I., Fomina L.P. Nitrogen diffusion along the layer boundaries after nitriding of multilayer materials. Izvestiya. Ferrous Metallurgy. 2024;67(3):318-324. https://doi.org/10.17073/0368-0797-2024-3-318-324
Introduction
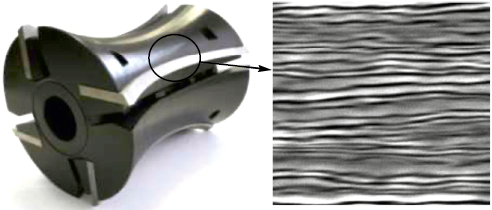
Currently various mechanical wood treatments by milling are widely used. The main unit of a router is the cutter. There are numerous cutters of different designs and geometries, but, in each case, they should be characterized by high strength and wear resistance, which can be achieved by chemical heat treatment [1]. The multilayer materials used after nitriding for manufacturing cutters can enable to enhance tool durability, preserve tool geometry, increase tool life, and improve processing performance due to a significantly hardened layer. Fig. 1 shows an example of such a cutter.
Fig. 1. Layout of a cutter from a multilayer material |
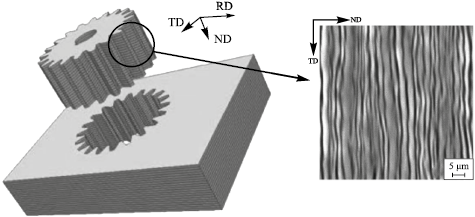
Additionally, multilayer materials can be used to manufacture gear wheels that also operate under wear (Fig. 2).
Fig. 2. Scheme of manufacturing a gear wheel from a multilayer material |
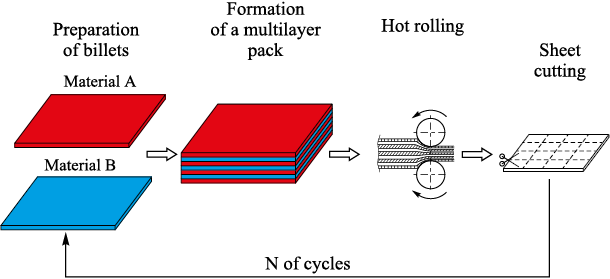
This structure can be obtained if steels with different crystalline structure are included in the initial composition [2 – 4]. The developed technological route (Fig. 3) enables to produce sheet billets, 2 to 10 mm thick [5].
Fig. 3. Scheme of technological route |
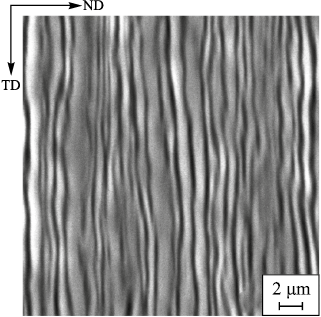
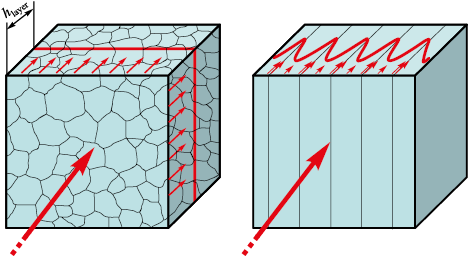
The material microstructure has a multilayer laminar structure with the layers, 100 to 0.8 µm thick. At the same time, the layers are characterized by crystallographic disorientation from 15 to 20°, which corresponds to the large-angle grain boundaries in the initial steels (Fig. 4) [6; 7].
Fig. 4. Typical microstructure of cross section of a multilayer material |
The works [8; 9] show that with an appropriate choice of steels included in the initial composite billet, the multilayer structure is preserved up to the temperature of 1000 °C, which corresponds to the temperature of hot pack rolling. In this case, the cross-section of the sheet billets has a structural orientation ready for chemical heat treatment (Fig. 5).
Fig. 5. Scheme of polyhedral and laminar structure |
Preliminary evaluation of nitriding the multilayer composition that includes AISI304 and AISI430 steels showed that the nitriding depth in the multilayer material is greater than its depth in AISI304 steel which is hard to nitride. The reason behind the increased thickness of the nitrided layer in multilayer materials may be the accelerated diffusion of nitrogen along the layer boundaries with subsequent saturation of layer volumes from their boundaries as from the diffusing element sources [10].
To thoroughly investigate the resulting effect, we used model compositions of multilayer materials containing steels of different compositions (grades). The following study objects were selected for nitriding: composites consisting of 1008 and AISI304 steels, as well as W108 and AISI304 steels. After hot pack rolling, we obtained 10-mm thick samples with 100 layers, the single layer being 100 μm thick.
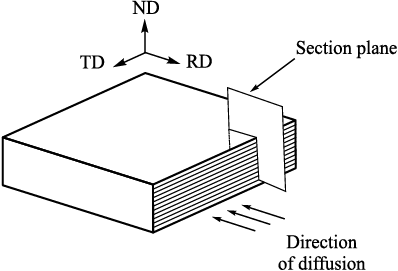
The samples of these compositions were nitrided, the nitriding surfaces being in all cases perpendicular to the rolling directions used to produce the multilayer samples. Nitriding was performed in the gas atmosphere containing 20 – 40 % ammonia dissociation products. We used two nitriding conditions with the following temperature and time parameters: 540 °C for 45 h and 580 °C for 25 h. To study the structure of the resulting nitrided layers and to determine their geometric characteristics, we prepared sections with the surfaces perpendicular to the rolling direction and parallel to the direction of nitrogen diffusion penetration that occurred in the course of nitriding (Fig. 6).
Fig. 6. Scheme of sample cutting for metallographic analysis |
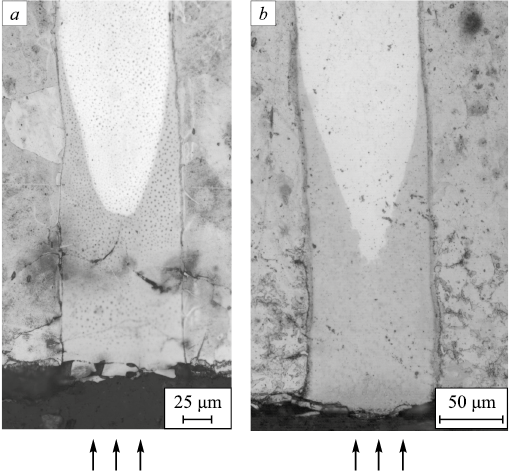
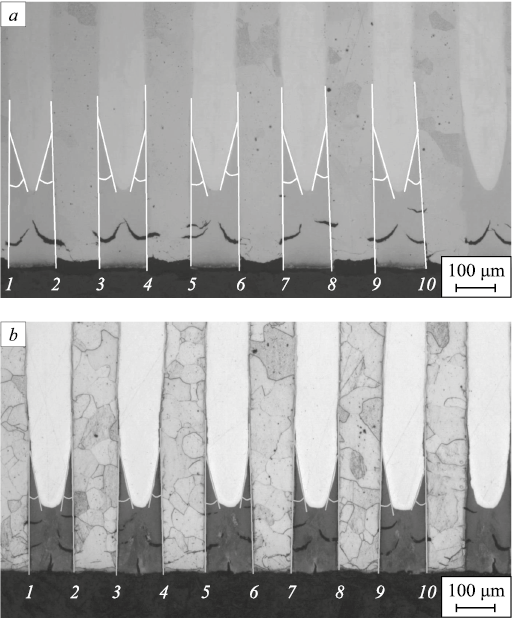
Fig. 7 shows the general view of diffusion profiles formed during nitriding for AISI304 and 1008 (a), AISI304 and W108 (b) compositions obtained after nitriding at 540 °C for 45 h.
Fig. 7. Microstructure of nitrided layer of composition based on steels: |
The micrographs show the concentration profile of diffusing nitrogen in AISI304 steel with a considerable nitrogen penetration depth (and a long nitrogen diffusion path, respectively) along the layer boundaries of the multilayer material. Inside the AISI304 component layer, for both cases, the nitrogen penetration distance shrinks with increasing distance from the interlayer boundary. The smallest depth of nitrogen penetration is about 100 μm from the outer surface of the samples and midway between the layers of neighboring components (midway between the layers of 1008 steel (a) and W108 steel (b)) inside the layer of AISI304 steel. This indicates that the source of nitrogen penetration into the volume of AISI304 steel during nitriding is the interface between the layers of the material. The diffusion along these boundaries occurs faster than that through the layer of AISI304 steel from the outer surface.
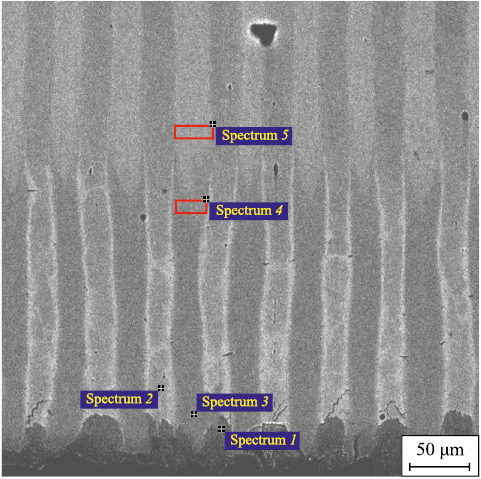
Nitrogen penetration into the layers of 1008 and W108 steels at this depth from the nitriding surface was not detected. This is proved by the results of electron microscopy and X-ray spectral analysis. There is practically no volume diffusion of nitrogen in 1008 and W108 steels due to low solubility of nitrogen in α-iron (maximum 0.1 wt. %), which is the main pathway of nitrogen diffusion in the ferrite phase [11]. The second reason inhibiting nitrogen movement in 1008 steel is the surface nitride formation consisting of nitrides (Fe2N), which is also proved by the results of electron microscopy (Fig. 8).
Fig. 8. Results of qualitative MRS analysis of the diffusion layer
Fig. 9. Concentration angle θ for determining the diffusion coefficient | |||||||||||||||||||||||||||||||||||||||||||||||||||
Thus, it can be concluded that the nitriding process in multilayer materials of this type occurs due to the fast nitrogen diffusion along the layer boundaries [12; 13].
It can be assumed that simultaneously nitrogen atoms migrate inside the AISI304 steel perpendicular to the interlayer boundary, which can be considered as an extended source of nitrogen diffusion. This steel is an austenitic grade steel and the solubility of nitrogen in austenite (γ-iron) is about 2.8 wt. %, therefore, diffusion saturation of AISI304 layers with nitrogen is possible. According to literature data [14], the diffusion coefficient of nitrogen in γ-iron at temperatures ranging from 500 to 600 °C is determined by the following equation
| \[{D_\gamma } = 4.6 \cdot {10^{ - 5}}\exp \left( { - \frac{{108{\rm{,}}474}}{{RT}}} \right){\rm{ }}{{\rm{m}}^{\rm{2}}}{\rm{/s}}.\] | (1) |
We used the Fisher model for calculating diffusion along grain boundaries in metallic samples to determine the diffusion permeability of layer boundaries of the multilayer material [15 – 18]. According to this model, the product of the diffusion coefficient Db along the grain boundary (layer boundaries in this case) and the boundary thickness δ, that is value of sδ Db, can be calculated by the formula [19]
| \[s\delta {D_b} = {(\pi t)^{1/2}}D_\gamma ^{3/2}{\rm{ct}}{{\rm{g}}^2}\theta ,\] | (2) |
where θ is the angle at the top of the component concentration profile (Fig. 7), which passes into the phase (layer) volume from the grain (layer) boundary; s is the ratio of boundary enrichment with atoms of the diffusing component, which can be estimated based on the dependence proposed in [20]:
| sx0 = 6.2 ± 4.5, | (3) |
where х0 is the volumetric concentration of the impurity in mole fractions.
If we assume that the enrichment of layer boundaries is mainly determined by the ferrite phase, since in accordance with the phase diagram it has the smallest Fe – N concentration of nitrogen, according to formula (3), the value of enrichment of layer boundaries shall equal s = 5·103.
It should be noted that the formula (2) is suitable for describing diffusion along the layer boundary when the diffusive removal of the component (nitrogen in this case) from the boundary into the bulk is one-sided – volume diffusion occurs only towards the layers of AISI304 steel. The experimental data shows that there is no diffusion of nitrogen toward the layers of 1008 steel (Fig. 10, a) or W108 steel (Fig. 10, b).
Fig. 10. Determination of the angles θ for calculating |
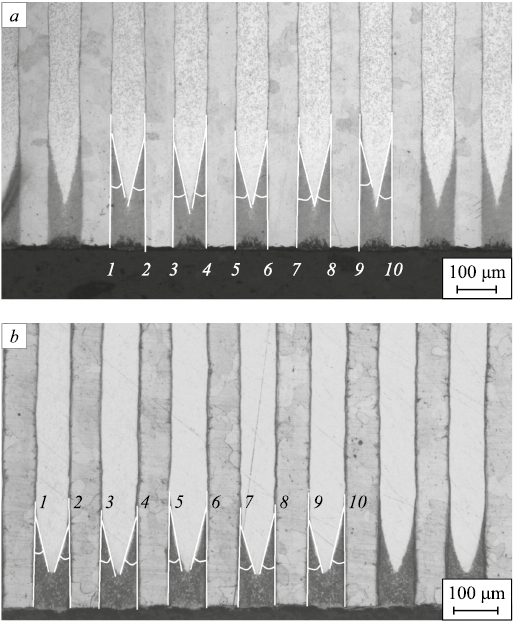
The values of angles θ required to calculate the diffusion coefficient Db along the layer boundaries were determined by analyzing micrographs of cross-sections of multilayer samples of both compositions after nitriding using two processing modes.
For the 1008 + AISI304 composition, Fig. 8, a shows the method for measuring these angles when nitriding is performed at 540 °C for 45 h and Fig. 10, b – when the operating parameters are 580 °C and 25 h. For the W108 + AISI304 composition, the similar procedure is shown in Fig. 11.
Fig. 11. Determination of the angles θ for calculating the product |
The Table presents the diffusion coefficients of nitrogen atoms along the layer boundaries for 1008 + AISI304 and W108 + AISI304 multilayer compositions obtained by analyzing experimental data as nitriding of these materials samples was investigated. The calculation assumes that the layer boundaries thickness δ is 10\(^–\)9 m.
Nitrogen diffusion coefficients along the layer boundaries Db , m2/s
| ||||||||||||||
Conclusions
The experimental study of nitriding the samples of multilayer metallic materials with alternating layers of two different steel grades revealed that the main mechanism behind the process is mass transfer (diffusion) of nitrogen atoms along the boundaries of the material layers.
The analysis of experimental data obtained while investigating cross sections of surface layers of two compositions of multilayer materials after nitriding using two modes enabled us to obtain the estimated values of nitrogen diffusion coefficients Db along layer boundaries. The Db values were 104 times higher than the volume diffusion coefficient of nitrogen in AISI304 steel under the same conditions.
The study showed that the nitriding depth in both multilayer compositions increased due to fast diffusion penetration of nitrogen atoms along the layer boundaries of multilayer materials.
References
1. Lakhtin Yu.M., Kogan Ya.D., Shpis G.I., Bemer Z. Theory and Technology of Nitriding. Moscow: Metallurgiya; 1991:320.
2. Huang B., Ishihara K.N., Shingu P.H. Preparation of high strength bulk nano-scale Fe/Cu multilayers by repeated pressing-rolling. Materials Science Letters. 2001;20:1669–1670. https://doi.org/10.1023/A:1012465117652
3. Yoshioka T., Yasuda M., Miyamura H., Kikuchi S., Tokumitsu K. Structure of Fe-Ag super-laminates fabricated by repeated rolling and mechanically alloyed Fe-Ag powder. Materials Science Forum. 2002;386–388:503–508. https://doi.org/10.4028/www.scientific.net/MSF.386-388.503
4. Saito Y., Utsunomiya H., Tsuji N., Sakai T. Novel ultra-high straining process for bulk materials development of the accumulative roll-bonding (ARB) process. Acta Materialia. 1999;47(2):579–583. https://doi.org/10.1016/S1359-6454(98)00365-6
5. Torizuka S. Present trend of the development of ultrafine-grained steels and its technology transfer. Materia Japan. 2006;45(6):438–443.
6. Kolesnikov A.G., Plokhikh A.I., Shinkarev A.S., Mironova M.O. Multilayer steel composition rolling peculiarities. Blanking Productions in Mechanical Engineering. 2013;(8):39–42.
7. Kolesnikov A.G., Plokhikh A.I., Komisarchuk Yu.S., Mikhal’tsevich I.Yu. A study of special features of formation of submicro- and nanosize structure in multilayer materials by the method of hot rolling. MiTOM. 2010;(6):44–49.
8. Tabatchikova T.I., Yakovlev I.L., Klyueva S.Yu. Structure and properties of a steel-based multilayer material produced by hot pack rolling. The Physics of Metals and Metallography. 2013;114(7):580–592.
9. Aryulin S.B., Khalipov I.V. Preparation of multilayer composite materials by hot rolling. Zagotovitel’nye proizvodstva v mashinostroenii. 2013;(7):31–35.
10. Plokhikh A.I. On the possibility of using metallic materials for machine parts hardened by chemical heat treatment. Izvestiya Volgogradskogo gosudarstvennogo tekhnicheskogo universiteta. 2013;(6(109)):13–17.
11. Bannykh O.A., Budberg P.B., Alisova S.P. State Diagrams of Binary and Multicomponent Systems Based on Iron. Moscow: Metallurgiya; 1986:224.
12. Polikevich K.B., Plokhikh A.I. Study of the process of nitriding in multilayer materials based on steel. IOP Conference Series: Materials Science and Engineering. 2019;683:012048. https://doi.org/10.1088/1757-899X/683/1/012048
13. Petelin A.L., Plokhikh A.I., Novikov A.A. The model of the layer boundary diffusion in multilayer materials. Defect and Diffusion Forum. 2015;363:142–147. https://doi.org/10.4028/www.scientific.net/DDF.363.142
14. Fedorov A.A. Diffusion of nitrogen in stainless steel. In: Technical Sciences in Russia and Abroad: Materials of the 3rd Sci. Conf. (Moscow, July 2014.). Moscow: Buki-Vedi; 2014:85-88. Available at URL: https://moluch.ru/conf/tech/archive/90/5561/ (accessed: 07.10.2024).
15. Bokshtein B.S., Kopetskii Ch.V., Shvindlerman L.S. Thermodynamics and Kinetics of Grain Boundaries in Metals. Moscow: Metallurgiya; 1986:224.
16. Boksteyn B.S., Nikolskiy G.S., Smirnov A.N. Grain boundary segregation of antimony in alloys of the cooper-antimony system. The Physics of Metals and Metallography. 1995;72:142–146.
17. Bokstein B.S., Straumal B.B. Diffusion in materials science and technology. In: Diffusive Spreading in Nature, Technology and Society. Springer, Cham; 2018:261–275. https://doi.org/10.1007/978-3-319-67798-9_13
18. Kaur I., Mishin Y., Gust W. Fundamentals of Grain and Interphase Boundary Diffusion. 3rd ed. John Wiley & Sons; 1995:536.
19. Mishin Y., Herzig Chr. Grain boundary diffusion: recent progress and future research. Materials Science and Engineering: A. 1999;260(1–2):55–71. https://doi.org/10.1016/S0921-5093(98)00978-2
20. Hondros E.D., Seah M.P. Segregation to interfaces. International Metals Reviews. 1977;22(1):262–301.
About the Authors
K. B. PolikevichRussian Federation
Kseniya B. Polikevich, Senior Lecturer of the Chair “Materials Science”
5/1 Baumanskaya 2-ya Str., Moscow 105005, Russian Federation
A. L. Petelin
Russian Federation
Aleksandr L. Petelin, Dr. Sci. (Phys.–Math.), Prof.; Prof. of the Chair of Physical Chemistry
5/1 Baumanskaya 2-ya Str., Moscow 105005, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
A. I. Plokhikh
Russian Federation
Andrei I. Plokhikh, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Materials Science”
5/1 Baumanskaya 2-ya Str., Moscow 105005, Russian Federation
L. P. Fomina
Russian Federation
Lyudmila P. Fomina, Cand. Sci. (Eng.), Assist. Prof.
16/2 Budennogo Ave., Moscow 105118, Russian Federation
Review
For citations:
Polikevich K.B., Petelin A.L., Plokhikh A.I., Fomina L.P. Nitrogen diffusion along the layer boundaries after nitriding of multilayer materials. Izvestiya. Ferrous Metallurgy. 2024;67(3):318-324. https://doi.org/10.17073/0368-0797-2024-3-318-324