Scroll to:
Increasing the corrosion properties of duplex steel with REM modification
https://doi.org/10.17073/0368-0797-2024-2-219-228
Abstract
Duplex stainless steels are a modern class of materials with a unique combination of high corrosion and mechanical properties. Due to this, they can be widely used in machine parts and aggregates in fields with aggressive oil and gas production conditions. One of the disadvantages of these materials is their tendency to local corrosion damage on non-metallic inclusions, other things being equal, formed during smelting and casting. To control the purity of steel in conditions of open induction smelting, it is effective to use modification with rare earth metals (REM). Therefore, the purpose of this work was to determine the optimal content of REM in duplex steel to increase corrosion properties. Thermodynamic modeling of the formation of nonmetallic inclusions in duplex corrosion-resistant steel S32750 was carried out and the results of calculations were compared with the experimental data. It is shown that there is an optimal concentration of REM at which contamination with inclusions is minimal due to favorable conditions for their removal, and with a further increase in consumption it increases due to coagulation of a large number of refractory oxides. Electrochemical tests were performed and parameters such as corrosion potential, pitting formation potential and the basis of pitting resistance of experimental steels were determined. Therefore, the corrosion properties of the investigated duplex steel are significantly improved when treated with REM. The electrochemical potentials of different types of inclusions are evaluated on a qualitative level. Based on the obtained results on corrosion resistance and contamination of the studied castings, the optimal amount of REM introduced for modifying inclusions is 0.05 % (0.65Ce + 0.35La).
Keywords
For citations:
Karasev V.S., Kodzhaspirov G.E., Fedorov A.S., Al’khimenko A.A., Zhitenev A.I. Increasing the corrosion properties of duplex steel with REM modification. Izvestiya. Ferrous Metallurgy. 2024;67(2):219-228. https://doi.org/10.17073/0368-0797-2024-2-219-228
Introduction
Currently, the operational activities of oil and gas companies are shifting towards reservoirs with more aggressive extraction conditions, which continuously increase and tighten the requirements for the properties and quality of materials used in equipment manufacturing. One of the unique materials that has been effectively used abroad for a long time but is only now gradually being implemented in the domestic industry is duplex stainless steels (DS) [1; 2]. Due to their high level of chromium, nickel, molybdenum, and nitrogen alloying, these steels possess resistance to general corrosion at the level of traditional austenitic steels but allow for significant strength characteristics due to the simultaneous existence of austenite and ferrite. At the same time, these steels are highly vulnerable to local types of corrosion, such as pitting and crevice corrosion [3; 4].
Under equal conditions, non-metallic inclusions (NMIs) are the sites of formation of local corrosion damage, which occur during melting and casting processes. Therefore, it is necessary to minimize their quantity or modify them into types that have a weak effect on corrosion properties. Publications have shown the degree of negative influence of different types of NMIs [5; 6], their quantity, size, and morphology [7 – 9] on the properties of finished products made from DS.
It is well known that various two-stage processes of special electrometallurgy, such as vacuum induction melting of ingots–electrodes followed by electroslag remelting, are used to produce high-quality steels and alloys [10 – 12]. However, this technology is quite expensive and is also not suitable for obtaining shaped castings, which make up a significant part of the range of machine parts and units for oil and gas production. Such castings are usually produced by melting in an open induction furnace with very limited conditions for refining the melt. One effective way to control inclusions in these conditions is to use rare earth metals (REM) as modifiers for NMIs. Thus, the introduction of REM can lead to the formation of different types of NMIs, both increasing [13 – 16] and decreasing [16 – 19] the corrosion resistance of highly alloyed steels and alloys.
Therefore, the aim of this study is to investigate the influence of the consumption and concentration of REM on the formation of NMIs in DS in an open induction furnace and to study their effect on the pitting corrosion resistance of DS.
Materials and methods
The study investigated duplex stainless steel of the austenitic-ferritic class, type S32750, with varying amounts of rare earth metals (REM), obtained in an open induction furnace with a capacity of 20 kg. After complete melting of the charge, the steel was deoxidized with silicon and manganese, and then with titanium. It was then held in the furnace for 1 min to average the chemical composition and poured into an 80 mm diameter cast iron mold with a capacity of 10 kg. A portion of REM was added to the crucible, and the remaining melt was poured into a ladle, from which the second ingot was cast. In the second melt, both ingots were produced using REM, but with different amounts. Thus, four ingots weighing 10 kg each were obtained (Table 1). Since the ratio of cerium to lanthanum in all ingots was the same and corresponded to the chemical composition of the used alloy, for convenience, the total amount of REM was used for each ingot in Table 1 and onwards.
Table 1. Chemical compositions of the studied duplex steels
| |||||||||||||||||||||||||||||||||||||||||||||||||
Therefore, the experiment was planned so that DS1 steel without REM and DS2 steel with a 0.02 % REM content were obtained in melt 1, while DS3 and DS4 steels with REM contents of 0.05 and 0.08 % respectively were obtained in melt 2. DS1 and DS3 steels were poured from the furnace spout, while DS2 and DS4 steels were poured using a ladle. Since it is believed that pouring through a ladle allows for intensified metal mixing at the moment of pouring and increases the inclusion floatation time, this order of obtaining the ingots allowed for studying the influence of REM consumption and casting technology in two melts, excluding the influence of other melting features.
To obtain the required amount of ferrite (50 %) and eliminate secondary phases formed during slow cooling of selected section ingots, samples were quenched in water after isothermal holding at 1100 °C. The contamination assessment of experimental steels with inclusions was carried out using ASTM E 1245 method by field-by-field cumulative method. The volumetric inclusion content V, %, average diameter by Feret d, μm, and maximum diameter of the largest inclusions dmax , μm, were evaluated. The chemical composition of NMI was determined by X-ray spectral microanalysis using the TESCAN Mira-3M scanning electron microscope.
Thermodynamic simulation of inclusion formation was conducted according to the methodology presented in [20 – 22]. For the calculation of primary inclusions, the initial oxygen content interacting with deoxidizers during their introduction was taken into account. To calculate the equilibrium type of NMI for each deoxidizer concentration during steel cooling, solubility surfaces of components in metal (SSCM) were calculated [20], using temperature-dependent equilibrium constants and first-order interaction parameters for each considered reaction [23 – 25]. When forming tertiary inclusions, liquation was taken into account according to the Scheil equation [26].
The resistance of steels to pitting corrosion was evaluated by electrochemical method using VersaStat Princeton Applied Research potentiostat. The tests were conducted in a 5 % aqueous solution of NaCl, acidified with acetic acid to pH = 3 at 22 °C. During the experiment, the following parameters were determined:
– the steady-state corrosion potential Esteady , which was recorded after holding without external polarization for 1 h;
– the corrosion potential Ecor , indicating the potential of the metal at which anodic and cathodic reactions are in equilibrium under polarization conditions;
– the pitting potential Epit , corresponding to the current at which pitting occurs as a result of local breakdown of metal passivity;
– the pitting resistance basis, calculated as the difference between the corrosion potential and the pitting potential.
Results and discussion
Thermodynamic simulation and assessment of NMIs
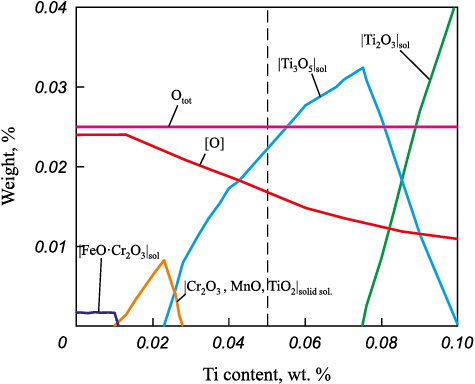
Fig. 1 shows the result of thermodynamic modeling of inclusion formation during deoxidation of experimental steel with titanium at 1550 °C. The initial oxygen content in equilibrium with non-deoxidized DS is 0.025 %. Upon introduction of titanium, up to a concentration of 0.01 %, deoxidation practically does not occur, the total oxygen content is equal to the sum of the amount of oxygen bound in a small number of primary inclusions and dissolved. In equilibrium with the melt, primary inclusions of the |FeO·Cr2O3 |solid sol. type are present. In the range of titanium concentrations from 0.010 to 0.023 %, the formation of a solid solution |Cr2O3 , MnO, TiO2 |solid sol. becomes possible, while the solubility of oxygen in the steel begins to decrease. With further increase in titanium concentration to 0.027 %, the formation of titanium oxide Ti3O5 occurs, and when the titanium concentration reaches 0.075 %, Ti2O3 inclusions are formed.
Fig. 1. Thermodynamic modeling of inclusions formation |
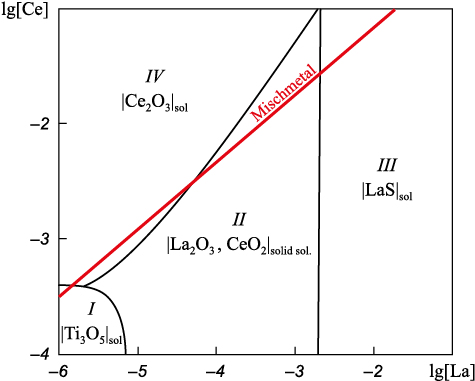
To track the changes in the equilibrium phase composition in DS when introducing REM, SSCM was calculated for the studied steel in the lg[Ce] – lg[La] coordinates (Fig. 2). Using this diagram, the combined influence of cerium and lanthanum on the type of formed non-metallic inclusions (NMI) can be assessed at a constant titanium concentration of 0.05 %, which corresponds to the experimental steels (Table 1, dashed line in Fig. 1). Let us consider the phases in equilibrium with the melt. In region I, the composition of the liquid metal is given, which is in equilibrium with solid particles |Ti3O5 |sol . Even with such a small amount of REM, the formation of inclusions |Cr2O3 , MnO, TiO2 |solid sol. , which appear during preliminary deoxidation with titanium, is completely suppressed. As the lanthanum content increases, the equilibrium phase becomes a solid solution of oxides |La2O3 , CeO2 |solid sol. ([La] ≥ 0.000007 %, region II), and with even higher concentration, lanthanum sulfide LaS is formed ([La] ≥ 0.0020 %, region III). The formation of Ce2O3 type inclusions is possible at a concentration of [Ce] ≥ 0.0004 wt. %. The SSCM also includes a line representing the ratio of cerium and lanthanum concentrations in the mischmetal. Thus, when increasing the amount of the added REM, the change in the equilibrium type of inclusions will occur along this line.
Fig. 2. Stability diagrams of nonmetallic inclusions (NMI) for the system |
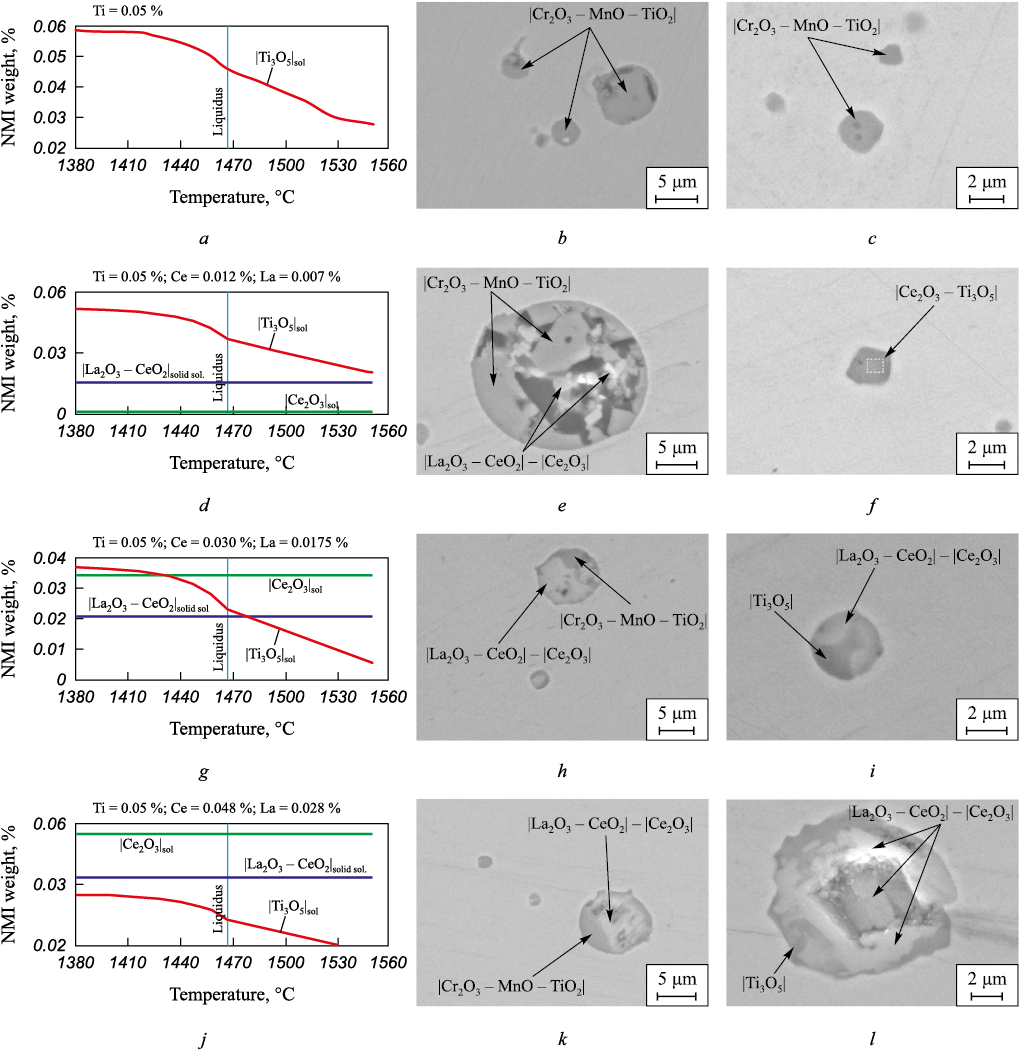
Since the formation of inclusions occurs not only during deoxidation and modification, but also during cooling and solidification, modeling of these stages has been carried out. The results of the calculations for all four steels are shown in Fig. 3, where images and phase composition of the found inclusions in these ingots are also included for convenience of analysis. Table 2 presents the results of the assessment of the volumetric fraction and sizes of NMIs.
Fig. 3. Thermodynamic modeling and NMIs in experimental steel ingots:
Table 2. Results of the assessment of contamination
|
In DS1 steel, pure titanium oxides Ti3O5 (Fig. 3, a) are formed initially as primary, and then as secondary and tertiary inclusions. However, in practice, complex oxides like Cr2O3 – MnO – TiO2 have been found in this steel. The formation of these inclusions is associated with the time point of introducing titanium into the melt, when microvolumes with different concentrations are formed due to its uneven distribution in the furnace, leading to the possible formation of various oxides (Fig. 1). This steel is the most contaminated with NMIs, with a volumetric fraction of 0.259 %, an average size of 1.8 µm, and the largest inclusion reaching 18 µm (Table 2).
When adding 0.02 % REM to DS2 steel, in addition to primary titanium oxides, the formation of a solution |Ce2O3 – La2O3 | becomes possible (Fig. 3, d). Similar NMIs have been found experimentally in the metal (Fig. 3, f). However, besides these, large inclusions of Cr2O3 – MnO – TiO2 have also been found in the same sample (Fig. 3, e). These inclusions, like in DS1 steel, are products of preliminary deoxidation that do not have time to recrystallize into equilibrium inclusions, resulting in their volumetric fraction in this steel being lower than in DS1 but still at a relatively high level (0.216 %). The average size of inclusions in this steel is almost the same, with the largest NMI size being 19 µm.
With an increase in REM concentration to 0.05 % (steel DS3), the fraction of primary lanthanum and cerium-based inclusions increases, while the number of forming titanium oxides decreases accordingly (Fig. 3, g). Experimentally, a large number of Ce2O3 – La2O3 inclusions and a significantly smaller number of Cr2O3 – MnO – TiO2 inclusions have been found in this steel. The contamination of NMIs in this steel is significantly lower, as primary REM inclusions are actively removed from the melt, with a fraction of 0.161 % and an average NMI size of 1.8 µm, with the largest inclusion size being 12 µm.
When introducing 0.08 % REM (DS4 steel), the formation of primary titanium oxides is completely suppressed, as all initial oxidization is removed due to the formation of REM inclusions (Fig. 3, j). In this case, titanium oxides are formed as secondary and tertiary inclusions, making their removal from the melt difficult. Two-phase NMI containing cerium and lanthanum with a small amount of Cr2O3 – MnO – TiO2 oxides were found in a real DS4 ingot (Fig. 3, k), as well as large inclusions based on cerium and lanthanum oxide and titanium oxide. By their morphology, it can be concluded that they are formed due to the coagulation of a large amount of refractory primary oxides (Fig. 3, l). The assessment results of contamination confirm this, as the volume fraction of NMI in this steel is higher than in DS3 steel, at 0.179 %, with an average size of 1.9 µm and the largest NMI size of 15 µm.
Analysis of the data in Table 2 also allows us to conclude that in the treatment of REM, the pouring technology (From spout or Through ladle) does not have a significant effect on the contamination of NMI.
Thus, thermodynamic simulation efficiently describes the observed types of NMI and their quantities in experimental steels. However, even in steels with increased REM content, non-equilibrium products of primary deoxidation by titanium are found, as the amount of primary inclusions in DS steel is so high that even 0.08 % REM for complete modification is insufficient.
Influence of NMIs on corrosion resistance
The results of the evaluation of the corrosion properties of the studied steels are presented in Table 3. The steady-state corrosion potential Esteady for the steel deoxidized only by titanium (DS1) is significantly lower than for the steels modified by REM. The addition of REM increased the value of Esteady , but there is no dependence between the specific amount of introduced REM and Esteady .
Table 3. Corrosion test results
|
The values of the equilibrium corrosion potential for the steels modified by REM are approximately the same and significantly lower than the corresponding parameter for the steel without REM. The pitting resistance potentials slightly increase from the first steel to the fourth. In order to fully describe the range of potentials at which the material exists in a passive state, the so-called pitting resistance basis is calculated. If the process of destruction of the oxide film begins at the corrosion potential, and its complete destruction occurs at the pitting potential, then the higher the value of the pitting resistance basis (∆E = Epit – Еcor ), the better the material’s resistance to pitting corrosion. Thus, the addition of REM significantly increased the resistance to pitting corrosion of all steels treated with mischmetal.
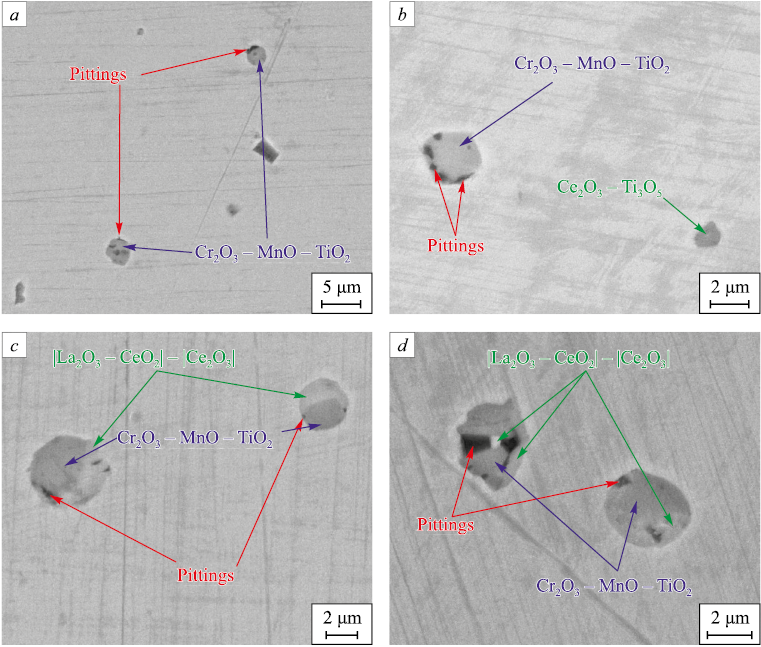
The investigation of the pitting initiation sites was carried out on polished samples after electrochemical testing (Fig. 4). In steels DS1 (Fig. 4, a) and DS2 (Fig. 4, b) without REM or weakly modified by REM, numerous pits were found on Cr2O3 – MnO – TiO2 inclusions. In steels DS3 (Fig. 4, c) and DS4 (Fig. 4, d), it was found that in two-phase inclusions, the part consisting of oxides based on Cr2O3 – MnO – TiO2 is primarily damaged, while the part based on cerium and lanthanum oxides is retained.
Fig. 4. NMIs in experimental steels after corrosion tests: |
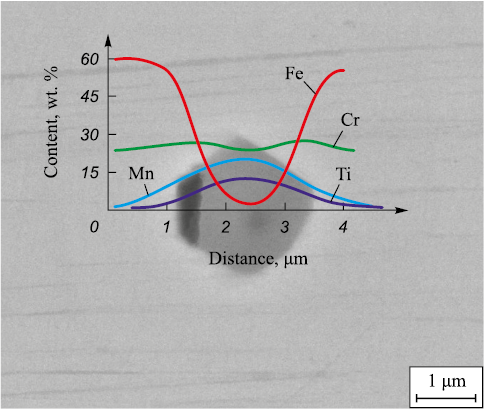
There are several hypotheses about the mechanisms of inclusion influence on pitting initiation and development. The first hypothesis is based on the difference in the coefficient of thermal expansion (CTE) between the inclusion and the matrix [6]. If the CTE of the inclusion is higher than that of the matrix, compressive stresses are generated upon cooling, leading to the formation of microvoids. Conversely, if the CTE of the inclusion is lower, tensile stresses are generated. However, in this case, this mechanism is not applicable as no porosity or microvoids were observed at the inclusion-matrix interface before testing (Fig. 3). The second hypothesis, proposed in [27], suggests the formation of chromium-depleted zones around oxide inclusions based on chromium. However, other studies [6; 28] that have investigated the distribution of chromium around oxides do not confirm this hypothesis. Moreover, in the present study, an investigation of the element distribution across the NMI section (Fig. 5) shows no depletion zone around the Cr2O3 – MnO – TiO2 inclusion. Since the diffusion relaxation of reactants around a growing inclusion in liquid metal occurs within a few seconds [29], and the reactant concentrations quickly equilibrate, depletion can only occur during the growth of the inclusion in solid metal, when the diffusion rate decreases by several orders of magnitude [30]. Due to the fact that Cr2O3 – MnO – TiO2 oxides are formed as primary inclusions (Fig. 1), the formation of a diffusion-depleted zone around them is impossible.
Fig. 5. Distribution of elements around Cr2O3 – MnO – TiO2 inclusion |
The third hypothesis is related to the dissolution of inclusions in the corrosive environment [7]. It is known that REM oxides form hydrolytically stable oxides in water [31]. However, these data should be cautiously applied to the considered electrolyte with a pH of 3. Direct studies on the nature of pitting formation (Fig. 4) only allow us to state the fact that cerium and lanthanum oxides have a higher potential in the chosen test medium, therefore, corrosion damage will primarily occur in the matrix. At the same time, pits were found specifically on the side of the Cr2O3 – MnO – TiO2 oxides in the vicinity of two-phase inclusions consisting of cerium and lanthanum oxides, and complex compounds of Cr2O3 – MnO – TiO2 oxides (Fig. 4, c, d). Therefore, the latter have a lower corrosion potential than the steel matrix. As shown above, as the REM content in the steel increases, the fraction of pure REM oxides increases while the fraction of complex NMIs, which are products of preliminary deoxidation, decreases. Therefore, in steel containing a higher amount of cerium and lanthanum oxide inclusions, the potential for pitting formation Epit and the basis ΔE are higher. The types of inclusions can be arranged in order of increasing potential in the selected electrolyte: Cr2O3 – MnO – TiO2 < steel matrix < La2O3 – CeO2/Ce2O3.
Conclusions
Taking into account the specific qualitative potential of the matrix and different types of inclusions, as well as the estimated quantity of inclusions and their sizes, the optimal amount of REM introduced for modifying inclusions in the studied DS is 0.05 %. At this content of REM, the best effect of modification can be achieved and the quantity and size of inclusions can be minimized, resulting in a significant improvement in corrosion properties. With a lower REM consumption, a large amount of primary oxidation products, which are more likely to form pitting, are preserved. On the other hand, with a significant increase in REM consumption, the volume fraction and size of inclusions increase due to intensified coagulation of primary refractory oxides. To increase the efficiency of modification and further reduce the required modifier consumption, measures should be taken to reduce the initial oxidation of the melt and decrease the quantity of primary inclusions.
References
1. Franci R., Byrne G. Duplex stainless steels – alloys for the 21st century. Metals. 2021;11(5):836. https://doi.org/10.3390/met11050836
2. Kazakov A.A., Zhitenev A.I., Fedorov A.S., Fomina O.V. Development of duplex stainless steel compositions. CIS Iron Steel Review. 2019;(2):20–26. https://doi.org/10.17580/cisisr.2019.02.04
3. Practical Guidelines for the Fabrication of Duplex Stainless Steel. 3nd ed. IMOA; 2014:63.
4. Patra S., Agrawal A., Mandal A., Podder A.S. Characteristics and manufacturability of duplex stainless steel: A review. The Indian Institute of Metals. 2021;74(5):1089–1098. https://doi.org/10.1007/s12666-021-02278-7
5. Fedorov A., Zhitenev A., Karasev V., Alkhimenko A., Kovalev P. Development of a methodology for the quality management of duplex stainless steels. Materials. 2022; 15(17):6008. https://doi.org/10.3390/ma15176008
6. Karasev V.S., Kodzhaspirov G.E. Investigation of the influence of deoxidation technology and hot plastic deformation on the evolution of non-metallic inclusions in super duplex steel. In: Int. Sci. Conf. “Modern Materials, Advanced Production Technologies and Equipment” SMPPTO-2023. 2023:92–93. (In Russ.).
7. Zhang Y., Hu Q., Dai M., Huang F., Frank Y.C., Liu J. Investigation of micro-electrochemical activities of oxide inclusions and microphases in duplex stainless steel and the implication on pitting corrosion. Materials and Corrosion. 2019;71(6):876–886. https://doi.org/10.1002/maco.201911335
8. Luo H., Li X., Dong C., Xiao K. Effect of solution treatment on pitting behavior of 2205 duplex stainless steel. Arabian Journal of Chemistry. 2017;10(1):90–94. https://doi.org/10.1016/j.arabjc.2012.06.011
9. Jeon S.H., Do H., Kim H., Park Y. Influence of oxygen content on the inclusion formation and pitting corrosion resistance of hyper duplex stainless steels. Materials Transactions. 2014;55(12):1872–1877. https://doi.org/10.2320/matertrans.M2014164
10. Utkina K.N., Levkov L.Ya., Fedorov A.S., Zhitenev A.I., Terekhin D.K., Kashirina Zh.K., Shipova E.V. Investigation of the influence of chemical composition and crystallization conditions on the formation of the structure of super duplex steels. In: Physico-Chemical Fundamentals of Metallurgical Processes (FKhOMP 2022). 2022:248–256. (In Russ.).
11. Dub V.S., Levkov L.Ya., Shurygin D.A., Tolstykh D.S., Klochai V.V., Коrzun E.L., Garchenko A.A. Electroslag remelting technology for contemporary engineering. retrospection and new possibilities. Metallurgist. 2018;62(5–6):511–520. https://doi.org/10.1007/s11015-018-0688-9
12. Orlov V., Levkov L., Dub V., Balikoev A., Shurygin D. New approach to development and manufacturing technologies of duplex steel. E3S Web of Conferences. 2019;121(6):04010. https://doi.org/10.1051/e3sconf/201912104010
13. Gupta C.K., Krishnamurthy N. Extractive metallurgy of rare earths. International Materials Reviews. 1992;37(1): 197–248. https://doi.org/10.1179/imr.1992.37.1.197
14. Kim S.T., Jeon S.H., Lee I.S., Park Y.S. Effects of rare earth metals addition on the resistance to pitting corrosion of super duplex stainless steel. Part 1. Corrosion Science. 2010;52(6): 1897–1904. https://doi.org/10.1016/j.corsci.2010.02.043
15. Wang X., Ha K., Zhou G., Wu H., Wu R. Effect of rare-earth on sulfides morphology and abrasive resistance of high sulfur steel. Materials for Mechanical Engineering. 2012; 36(5):33–37.
16. Mei Z., Wan T., Lou D. Influence of RE modifier on as-cast grain refinement of super-low carbon cast steel. Special Casting & Nonferrous Alloys. 2002;(2):3–4.
17. Yan H.H., Hu Y., Zhao D.W. Microstructure and properties of as-cast 30Mn steel. AIP Advances. 2018;8(12):125128. https://doi.org/10.1063/1.5065444
18. Wang H., Wang A., Li C., Yu X. Xie J., Liang T., Liu C. Effects of rare earth metals on microstructure, mechanical properties, and pitting corrosion of 27% Cr hyper duplex stainless steel. Reviews on Advanced Materials Science. 2022;61(1):873–887. https://doi.org/10.1515/rams-2022-0284
19. Ha H.Y., Park C.J., Kwon H.S. Effects of misch metal on the formation of non-metallic inclusions and the associated resistance to pitting corrosion in 25% Cr duplex stainless steels. Scripta Materialia. 2006;55(11):991–994. https://doi.org/10.1016/j.scriptamat.2006.08.014
20. Mikhailov G.G., Leonovich B.I., Kuznetsov Yu.S. Thermodynamics of Metallurgical Processes and Systems. MISIS; 2009:520. (In Russ.).
21. Kazakov A.A., Kovalev P.V., Ryaboshchuk S.V., Mileikovskii A.B., Malakhov N.V. Investigation of the temperature-time nature of nonmetallic inclusions in order to improve the metallurgical quality of high-strength pipe steels. Chernye metally. 2009;(12):5–11. (In Russ.).
22. Kazakov A.A., Urazgil’deev A.Kh., Gusev A.A. Algorithmic model of formation of nonmetallic inclusions in liquid and solidifying steel. Izv. AN SSSR. Metally. 1989;(3):60–65. (In Russ.).
23. Kulikov I.S. Deoxidation of Metals. Metallurgiya; 1975:504. (In Russ).
24. Turkdogan E.T. Physical Chemistry of High-Temperature Processes. Academic Press; 1980:447.
25. Grigoryan V.A., Stomakhin A.YA., Ponomarenko A.G., etc. Physico-Chemical Calculations of Electric Steelmaking Processes: Tutorial for Universities. Metallurgiya; 1989:288. (In Russ.).
26. Kurz W., Fisher D., Rappaz M. Fundamentals of Solidification. Trans. Tech. Publications; 2023:353.
27. Ha H., Park C., Kwon H. Effects of non-metallic inclusions on the initiation of pitting corrosion in 11% Cr ferritic stainless steel examined by micro-droplet cell. Corrosion Science. 2007;49(3):1266–1275. https://doi.org/10.1016/j.corsci.2006.08.017
28. Meng Q., Frankel G.S., Colijn H.O., Goss S.H. Stainless-steel corrosion and MnS inclusions. Nature. 2003;424: 389–390. https://doi.org/10.1038/424389b
29. Kang Y., Lee H. Thermodynamic analysis of Mn-depleted zone near Ti oxide inclusions for intragranular nucleation of ferrite in steel. ISIJ International. 2010;50(4):501–508.
30. Kazakov A.A. Non-metallic inclusions in steel. Theory and its applications. In: Advanced Materials. TSU; 2017:203-275. (In Russ.).
31. Rabinovich V.A., Khavin Z.Ya. Brief Chemical Reference. 2nd ed. Leningrad: Khimiya; 1978:392. (In Russ.).
32.
About the Authors
V. S. KarasevRussian Federation
Vladimir S. Karasev, Engineer of the Scientific and Educational Center “Severstal-Polytech”
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
G. E. Kodzhaspirov
Russian Federation
Georgii E. Kodzhaspirov, Dr. Sci. (Eng.), Prof. of the Higher School of Physics and Technology of Materials
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
A. S. Fedorov
Russian Federation
Aleksandr S. Fedorov, Engineer of the Scientific and Technological Complex “New Technologies and Materials”
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
A. A. Al’khimenko
Russian Federation
Aleksei A. Al’khimenko, Director of the Scientific and Technological Complex “New Technologies and Materials”
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
A. I. Zhitenev
Russian Federation
Andrei I. Zhitenev, Cand. Sci. (Eng.), Head of Technological Projects, Directorate for Research and Development of New Products, Department of “Electrotechnical Steels”
2 Metallurgov Sqr., Lipetsk 398040, Russian Federation
Review
For citations:
Karasev V.S., Kodzhaspirov G.E., Fedorov A.S., Al’khimenko A.A., Zhitenev A.I. Increasing the corrosion properties of duplex steel with REM modification. Izvestiya. Ferrous Metallurgy. 2024;67(2):219-228. https://doi.org/10.17073/0368-0797-2024-2-219-228
JATS XML