Scroll to:
Effect of yttrium additions on microstructure and corrosion resistance of Incoloy 825 alloy
https://doi.org/10.17073/0368-0797-2024-1-83-88
Abstract
The work is devoted to the study of the effect of microalloying with yttrium (Y) additives to improve the corrosion resistance of Incoloy 825 superalloy. The influence of Y on microstructure was evaluated by metallographic methods using optical and scanning electron microscopes, resistance to pitting and intergranular corrosion was evaluated by electrochemical and chemical methods of analysis. The paper describes changes in the structure, phase composition and hardness of cast samples with yttrium content of 0, 0.01, 0.05 and 0.1 wt. %. The obtained data correlate with the results of thermodynamic calculations of phase formation during crystallization. The influence of additions on the structure after strain hardening was investigated. Small addition (up to 0.01 wt. %) promotes increase of mobility of recrystallized grain boundaries. With increasing Y amount, the grain size decreases and hardness increases. It is shown that the greatest deoxidizing ability is observed at small additions of Y in the amount up to 0.01 wt. %, while the total amount of dissolved [O] decreased five times. Increasing the Y content reduces the ability to remove heavy inclusions from the melt, resulting in an increase in the proportion of oxide inclusions. The effect of additives on nitrogen [N] was not observed, and the volume fraction of nitride inclusions did not change, but the size of nitride inclusions decreased and the character of their distribution changed to uniform than in the alloy without Y. The results of pitting and intergranular fracture resistance tests showed that Y is an element that can be used to improve the corrosion properties of Incoloy 825 alloy. The best combination of resistance to the two types of corrosion was observed for the 0.01 wt. % Y sample.
Keywords
For citations:
Salynova M.A., Uglunts T.V., Tolochko O.V. Effect of yttrium additions on microstructure and corrosion resistance of Incoloy 825 alloy. Izvestiya. Ferrous Metallurgy. 2024;67(1):83-88. https://doi.org/10.17073/0368-0797-2024-1-83-88
Introduction
The influence of catalysts, pressure, and temperature during oil refining induces alterations in the chemical composition of oil. Throughout the refining process, the constituents within crude oil undergo reactions facilitated by catalysts. The resultant compounds can cause detrimental effects on purification equipment, leading to severe corrosion [1].
This corrosion substantially diminishes process efficiency, necessitating careful selection of materials for equipment components. Superalloys, such as the foreign Incoloy 825 alloy, are recommended for manufacturing equipment [1 – 3].
The Incoloy 825 alloy possesses a distinctive set of properties, including resistance to stress corrosion, pitting in active media, and intergranular corrosion [3]. Nevertheless, the ever-increasing demands for alloy properties prompt researchers to explore new methods and avenues for enhancing alloy characteristics [4 – 6].
Several studies highlight the beneficial influence of rare earth metals (REMs) on the microstructural characteristics and mechanical properties of alloys, owing to their heightened sensitivity to oxygen and sulfur [7 – 12]. Cerium and yttrium are the most commonly utilized REMs in nickel superalloys, positively impacting mechanical properties at elevated temperatures through the mechanism of solid solution hardening [12 – 16]. Additionally, they contribute to the modification of carbides and eutectic phases [17; 18].
As per a patent [19], REM additions in conjunction with calcium and/or magnesium result in significant desulfurization of Ni – Cr alloys, facilitating sustained inhibition of hot workability deterioration in the low-temperature range. However, REMs are susceptible to oxidation; hence, they should be added to the pre-deoxidized melt in restricted amounts (0.010 to 0.074 %). As the content increases, a large number of finely dispersed, high-density rare earth metal (REM) oxide nonmetallic inclusions (NMIs) are formed. NMIs are difficult to remove from the melt and contribute to a reduction in material impact strength.
Similar conclusions were drawn by the authors of [13]. They found that the microstructure of a Ni–16Mo–7Cr–4Fe nickel-based alloy was significantly enhanced by the addition of 0.05 wt. % Y. The alloy’s hardness and strength increased as solid solution hardening occurred with yttrium. However, when the yttrium content in the alloy exceeded 0.43 wt. %, the refractory intermetallic phase Ni17Y2 could emerge and grow, leading to a sharp deterioration in mechanical properties.
The addition of yttrium at 0.05 wt. % to the IN – 13C alloy positively impacted its high-temperature tensile properties [15]. Furthermore, in the as-cast condition, the ingot’s crystallization texture was refined.
Research interest is currently focused on the REM impact on the corrosion properties of steels and alloys. However, there is no unanimous opinion regarding the influence of specific rare-earth elements on certain alloy properties. Most studies are dedicated to investigating the effect of the rare-earth element yttrium on the resistance of the Incoloy 825 alloy to pitting and intergranular corrosion.
Materials and methods
To investigate the impact of yttrium microalloying on the corrosion and mechanical properties of Incoloy 825 alloy, a series of batches with varying additive content was melted. The experimental alloys were melted using a 15 kW open induction furnace in quartz crucibles. The chemical compositions of the alloy samples under investigation are provided in the Table.
Calculated chemical composition of the samples and actual content
|
Following melting, the ingots underwent homogenization and were subsequently forged on a press within a temperature range of 970 to 1150 °C. The forged samples were then annealed at a temperature of 960 ± 10 ℃ for 1 h, followed by quenching in water. This procedure aligns with the standard technology used for producing parts from Incoloy 825 alloy.
The microstructures of the stabilized alloys were analyzed using scanning electron microscopy (SEM) in backscatter mode, utilizing a Tescan Mira SEM instrument. Additionally, the phase composition was examined using a DRON-7 diffractometer.
To evaluate the resistance of the compositions to general and pitting corrosion, polarization curves were generated. These curves were instrumental in determining the steady-state corrosion potential (Ecor ) and pitting breakdown potential (Epit ) [20]. Imaging was conducted in a 5 % NaCl electrolyte acidified with acetic acid to achieve a pH of 3.00 ± 0.02 within an open, aerated electrochemical cell at room temperature. The polarization curve was obtained within potential ranges from –450 to 1100 mV at a scan velocity of 0.16 mV/s. The standard silver-chloride electrode (AgCl) served as the reference electrode during testing.
The alloys underwent intergranular corrosion (IGC) testing following ASTM G28 [21] guidelines. Testing involved immersing the samples in a boiling solution comprising 50 % H2SO4 and iron (III) sulfate for a duration of 120 h. Prior to testing, the samples were sensitized at 700 ℃ for 1 h. IGC values were determined through mass loss analysis and metallographic methods, with the depth of corrosion damage also being measured.
Analysis of microstructural features
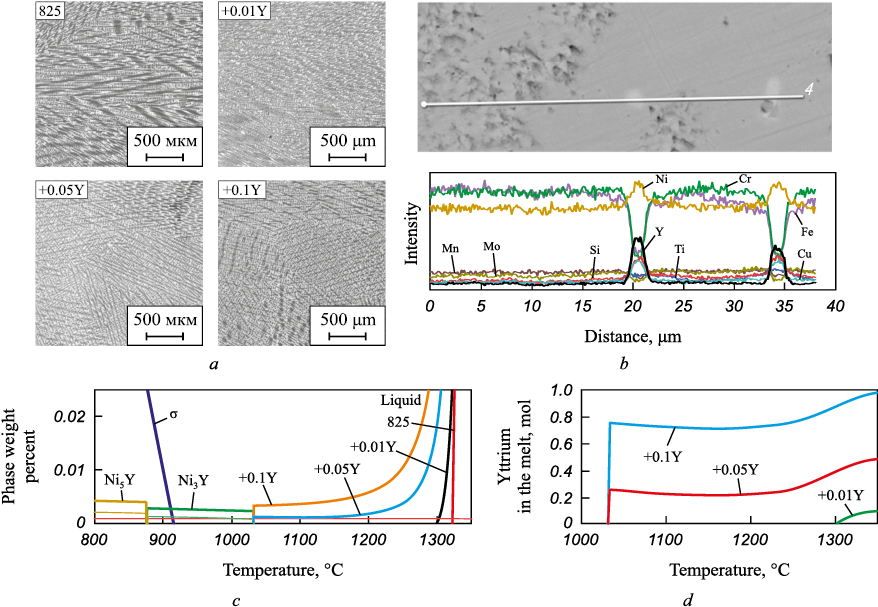
Fig. 1, a displays microstructure images of cast samples with varying amounts of yttrium. These images were captured at a distance equivalent to 1/2 the ingot radius. The original alloy sample, devoid of yttrium additions, exhibits a region characterized by columnar crystals. However, upon the addition of yttrium at 0.01 wt. %, the dendritic structure of the alloy begins to refine during solidification. This effect becomes more pronounced with increasing additive content, resulting in a shift from predominantly columnar to predominantly equiaxial dendritic structure. It is noteworthy that subsequent thermomechanical processing of the alloy necessitates an equiaxial structure within the ingot, as it reduces the risk of defects during forging operations. Consequently, an equiaxial structure is deemed optimal. Following yttrium microalloying, the Vickers hardness value of the samples increases from 140 to 160 HV.
Fig. 1. Images of microstructure of ingots (a): interdendritic segregation of yttrium |
SEM analysis of the cast samples revealed finely dispersed yttrium-rich intermetallic inclusions, measuring up to 2 μm in diameter, with nickel present in the interdendritic regions (Fig. 1, b). Thermodynamic calculations supported the notion that such inclusions could precipitate from the melt (Fig. 1, c, d). It was observed that even small additions of yttrium (0.01 wt. %) expanded the two-phase region during crystallization. With increased additive content, the solidus temperature notably decreases, resulting in oversaturation of the melt with yttrium. This facilitates the formation of high-temperature Ni3Y inclusions, the abundance of which is contingent upon the quantity of yttrium added. The heightened hardness of ingots is likely attributable to these refractory inclusions.
Many researchers examining the impact of REMs on the microstructure of superalloys have analyzed cast samples and arrived at similar conclusions [4; 7]. However, there has been scarce investigation into the impact of REMs on the structure of alloys after strain hardening [12].
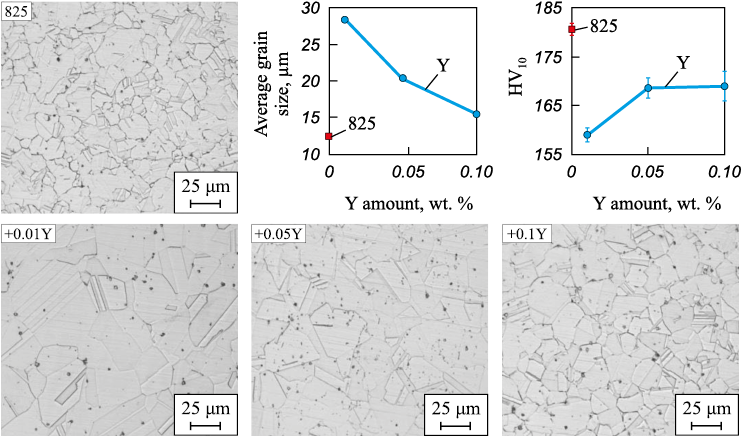
Fig. 2 illustrates how the size of the austenitic grain of samples, following forging and annealing, varies with the amount of added yttrium. In the sample containing the lowest yttrium additive content (0.01 wt. %), the grain size is 2.2 times larger than in the original alloy sample, measuring 28.4 µm. Concurrently, the hardness value decreases by 15 %. With an increase in the amount of additive, there is a refinement of the recrystallized austenitic grain and an associated increase in hardness (Fig. 2).
Fig. 2. Dependence of austenitic grain size of the samples after deformation |
As previously noted [4 – 6], the high sensitivity of REM elements to oxygen and sulfur contributes to a reduction in impurity content at grain boundaries, effectively “purifying” them and enhancing the mobility of grain boundaries, thereby increasing their size. This underscores how even a minute concentration of impurities can significantly influence the mobility of grain boundaries.
These findings align well with the total oxygen content values in the samples (as indicated in the Table) obtained through gas analysis, as well as with the assessment of NMIs in the samples.
Assessment of non-metallic inclusions and corrosion resistance
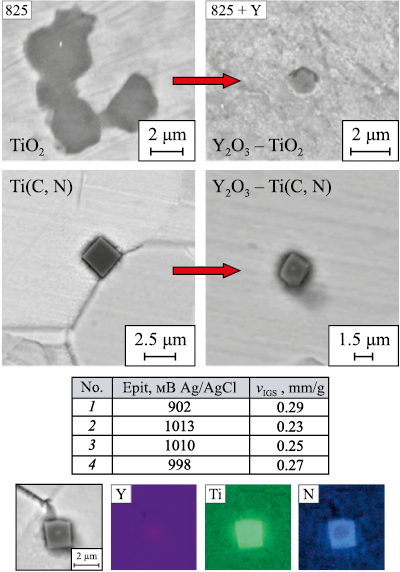
Yttrium additions play a significant role in reducing the oxygen content and deoxidizing the melt. Even with small yttrium additives, the total oxygen content decreased by a factor of five (as indicated in the Table), with the volume fraction of oxide NMIs amounting to 0.0055 ± 0.004 %. Following the addition of yttrium, the composition and size of oxide inclusions transition to complex oxides of yttrium and titanium. As the additive content increases, the total oxygen content and inclusion values grew to 0.093 ± 0.033 %, yet they remain lower than those in the original alloy sample, where the oxide volume fraction is 0.13 ± 0.05 %.
While yttrium does not influence the nitrogen content in the alloy (as shown in the table), and consequently, the volume fraction of carbonitride Ti(C, N) NMIs, it does alter the morphology of these inclusions. In samples with yttrium microalloying, complex oxycarbonitride inclusions of the Y2O3 − Ti(C, N) system are formed. In this scenario, finely dispersed deoxidation products, namely yttrium oxides, serve as a substrate for carbonitride formation (Fig. 3).
Fig. 3. Variation of non-metallic inclusions and corrosion resistance |
The results of electrochemical and chemical corrosion resistance tests are depicted in Fig. 3. An yttrium additive (0.01 wt. %) increased the pitting breakdown potential by 13 % and reduced the intergranular fracture rate by 20 %. However, as the additive amount increases, so does the proportion of oxide inclusions rich in titanium and yttrium, which diminishes the effect of melt stabilization by titanium and accounts for the decreased IGC resistance.
Conclusions
In this study, we investigated the relationship between changes in the microstructure of cast samples after strain hardening, NMI characteristics and corrosion resistance of Incoloy 825 alloy with different content of yttrium additives.
Following stabilizing annealing, there were notable changes in the phase composition. Introduction of yttrium additives led to an expansion of the two-phase region during crystallization. Additionally, the liquid portion of the dendritic cell became oversaturated with yttrium, which transformed into high-temperature inclusions of Ni3Y during solidification, as confirmed by calculations.
In the state, increasing yttrium additions resulted in a shift in solidification structure from predominantly columnar to predominantly equiaxial. Simultaneously, pressure treatment of the alloys proved to be more effective, yielding no cracks.
Yttrium acts as an active deoxidizing element; its impact is more pronounced with smaller additive amounts, as confirmed by gas analysis and measurement of grain size in samples after forging and annealing.
Yttrium additives altered the composition of NMIs in the alloy to yttrium-modified complex inclusions, characterized by a more rounded shape and smaller size. Such inclusions demonstrated enhanced chemical resistance in aggressive media during corrosion testing, with the sample containing 0.01 wt. % Y exhibiting the best corrosion resistance. Due to the increased grain size imparted by this composition, the alloy possesses higher ductility, necessitating the selection of a new heat treatment regimen.
References
1. Reed R.C. The Superalloys: Fundamentals and Applications. Cambridge University Press; 2008:392.
2. Mankins W.L., Lamb S. Nickel and nickel alloys. In: ASM Metals Handbook. Vol. 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials. Materials Park, OH, USA: ASM International; 1990:428–445.
3. ASTM B564 Standard Specification for Ni-Fe-Cr-MoCu Alloy (UNS N08825 and UNS N08221) Rod and 108 Bar. West Conshohocken, PA: ASTM International; 2011.
4. Botinha J., Krämer J., Genchev G., Bosch C., Alves H. Effect of sensitization on the corrosion resistance of an advanced version of alloy UNS N08825. In: Proceedings of the NACE Int. Corrosion Conf., Nashville, TN, USA, 24–28 March 2019:1–12.
5. Shoemaker L., Crum J. Processing and fabricating alloy 825 for optimized properties and corrosion resistance. In: Proceedings of the NACE Corrosion Conf., Houston, TX, USA, 13–17 March 2011:1–13.
6. Raymond E.L. Mechanisms of sensitization and stablization of Incoloy nickel-iron-chromium alloy 825. Corrosion. 1968;24(6):180–188. https://doi.org/10.5006/0010-9312-24.6.180
7. Li J.-P., Zhang H.-R., Gao M., Li Q.-L. Mechanism of yttrium in deep desulfurization of NiCoCrAlY alloy during vacuum induction melting process. Rare Metals. 2022;41(3): 218–225. https://doi.org/10.1007/s12598-018-1103-1
8. Du T., Wang L., Liu A., Wu Y., Zhang Y. Thermodynamics and phase equilibria for cerium and yttrium in the presence of oxygen and sulphur in nickel-base solutions. Journal of Alloys and Compounds. 1993:193(1–2):38–40. https://doi.org/10.1016/0925-8388(93)90303-5
9. Ishii F., Ban-Ya S. Equilibrium between yttrium and oxygen in liquid iron and nickel. ISIJ International. 1995;35(3): 280–285. https://doi.org/10.2355/isijinternational.35.280
10. Dan T., Gunji K. Deoxidation characteristics and shape modification of deoxidation products with Al-Ce and Al-Y complex deoxidizers. Tetsu-to-Hagané. 1982;68(14):1915–1921. https://doi.org/10.2355/tetsutohagane1955.68.14_1915
11. Kwon S., Kong Y., Park J. Effect of Al deoxidation on the formation behavior of inclusions in Ce-added stainless steel melts. Metals and Materials International. 2014;20(5): 959–966. https://doi.org/10.1007/s12540-014-5022-x
12. Fujikawa H., Morimoto T., Nishiyama Y., Newcomb S. The effects of small additions of yttrium on the high-temperature oxidation resistance of a Si-containing austenitic stainless steel. Oxidation of Metals. 2003;59(1):23–40. https://doi.org/10.1023/A:1023061814413
13. Palleda T., Banoth S., Tanaka M., Murakami H., Kakehi K. The role of yttrium micro-alloying on microstructure evolution and high-temperature mechanical properties of additively manufactured Inconel 718. Materials & Design. 2023;225:111567. https://doi.org/10.1016/j.matdes.2022.111567
14. Zhou P.J., Yu J.J., Sun X.F., Guan H.R., Hu Z.Q. Roles of Zr and Y in cast microstructure of M951 nickel-based superalloy. Transactions of Nonferrous Metals Society of China. 2012;22(7):1594–1598. https://doi.org/10.1016/S1003-6326(11)61361-7
15. Kang D.S., Koizumi Y., Yamanaka K., Aoyagi K., Bian H., Chiba A. Significant impact of yttrium microaddition on high temperature tensile properties of Inconel 713C superalloy. Materials Letters. 2018;227:40–43. https://doi.org/10.1016/j.matlet.2018.03.106
16. Chen L., Ma X., Wang L., Ye X. Effect of rare earth element yttrium addition on microstructures and properties of a 21Cr-11Ni austenitic heat-resistant stainless steel. Materials & Design. 2011;32(4):2206–2212. https://doi.org/10.1016/j.matdes.2010.11.022
17. Guimarães A., Silveira R., Almeida L., Araujo L., Farina A., Dille J. Influence of yttrium addition on the microstructural evolution and mechanical properties of superalloy 718. Materials Science and Engineering: A. 2020;776:139023. https://doi.org/10.1016/j.msea.2020.139023
18. Cao S., Yang Y., Chen B., Liu K., Ma Y., Ding L., Shi J. Influence of yttrium on purification and carbide precipitation of superalloy K4169. Journal of Materials Science & Technology. 2021;86:260–270. https://doi.org/10.1016/j.jmst.2021.01.049
19. Tomio Yu., Sigara M. Ni-Cr alloy material and seamless oilfield pipe products made from it. Patent RF no. 2630131. MPK В21С 1/00. Bulleten’ izobretenii. 2017;(25). (In Russ.).
20. ISO 17475:2005. Corrosion of Metals and Alloys – Electrochemical Test Methods – Guidelines for Conducting Potentiostatic and Potentiodynamic Polarization Measurements.
21. ASTM. G 28 Standard Test Methods for Detecting Susceptibility to Intergranular Corrosion in Wrought, Nickel-Rich, Chromium-Bearing Alloys. West Conshohocken, PA, USA: ASTM International; 2015.
About the Authors
M. A. SalynovaRussian Federation
Mariya A. Salynova, Engineer
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
T. V. Uglunts
Russian Federation
Tigran V. Uglunts, Engineer
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
O. V. Tolochko
Russian Federation
Oleg V. Tolochko, Dr. Sci. (Eng.), Prof., Leading Researcher
29 Politekhnicheskaya Str., St. Petersburg 195251, Russian Federation
3 Lotsmanskaya Str., St. Petersburg 190121, Russian Federation
Review
For citations:
Salynova M.A., Uglunts T.V., Tolochko O.V. Effect of yttrium additions on microstructure and corrosion resistance of Incoloy 825 alloy. Izvestiya. Ferrous Metallurgy. 2024;67(1):83-88. https://doi.org/10.17073/0368-0797-2024-1-83-88