Scroll to:
Effect of hydrogen on steels in hydrogen sulfide-containing and other environments at gas facilities
https://doi.org/10.17073/0368-0797-2024-1-53-64
Abstract
The impact of hydrogen sulfide raw materials on steel equipment and pipelines is known and is associated not only with internal corrosion processes, but also with the hydrogenation of carbon and low-alloy steels used. Penetration of hydrogen into steel can lead to the loss of its strength properties and subsequent destruction of gas pipelines operated under high pressure conditions. The manifestations of cracking characteristic of hydrogen sulfide environments, which are a consequence of the penetration of hydrogen into steel, are the most dangerous from the point of view of the safety and reliability of the operation of facilities for the production and transportation of corrosive gas. The effect of H2S on the decrease in ductility of the main types of structural steels was studied based on the results of simulation tests. The formation of blisters (bloatings) and cracks on the surface of steels due to the effect of hydrogen on steel was recorded. The study of the phase composition and properties of corrosion products was carried out in order to assess their possible influence on the processes of steel hydrogenation. The formation of evenly distributed on the surface and the densest corrosion deposits will hinder both the corrosion processes and the penetration of hydrogen into steel. A decrease in the plastic properties of steel is also observed when exposed to hydrogen, which can be transported both separately and together with methane through the main gas pipelines. The main possible means of protecting steels that are unstable to hydrogenation is the use of corrosion inhibitors. It was established that the most effective corrosion inhibitors with rational technologies of application and dosage can protect steels from penetration of hydrogen into them and their destructive effect.
Keywords
For citations:
Kantyukov R.R., Zapevalov D.N., Vagapov R.K. Effect of hydrogen on steels in hydrogen sulfide-containing and other environments at gas facilities. Izvestiya. Ferrous Metallurgy. 2024;67(1):53-64. https://doi.org/10.17073/0368-0797-2024-1-53-64
Introduction
The reliable and secure functioning of infrastructure is essential for the effective exploitation of oil and gas reserves. Corrosive components like carbon dioxide (СО2) and hydrogen sulfide (H2S), especially when water is present (either formation water or from condensation), can lead to widespread or localized corrosion and fractures [1].
Environments containing H2S are notably aggressive, leading to the hydrogenation of steel and causing both localized and general hydrogen sulfide corrosion (HSC) [2]. The permeation of hydrogen into steel poses a heightened risk, and thus the durability of steel structures and pipelines in high H2S environments is evaluated with consideration of the potential for hydrogen induced cracking [3].
It is important to recognize that the operational conditions at oil and gas facilities vary depending on whether the primary extracted fluids are oil or gas/gas condensate. These differences affect the types of corrosion that occur within pipelines. Previous research [4] has indicated that a particularly hazardous form of internal corrosion in gas pipelines involves localized corrosion at the top of the pipe where moisture condenses, collects, and then flows along the lower external curve of the pipe.
The Orenburg and Astrakhan gas condensate fields are examples where conditions conducive to HSC complications are present [2]. These sites are not unique in their susceptibility to HSC and hydrogenation issues. Moreover, pipeline systems for the reinjection of “sour gases” (a mixture of H2S and СО2 extracted from fluids during processing) are being developed at the Astrakhan field for disposal and enhanced condensate recovery in later production stages [5]. The H2S concentration in the gases transported through these pipelines will be considerably higher than in the initial extraction.
There is also growing interest in hydrogen as a fuel and its combined transportation with natural gas through main pipelines, as well as in the storage of hydrogen in underground facilities [6 – 7]. The use of hydrogen raises inevitable concerns regarding its explosive nature and its effect on the mechanical integrity of pipelines made from carbon/low alloy steel.
Some studies [8] suggest that hydrogen-containing gases could impact steel in a manner similar to H2S: atomic hydrogen is generated, diffuses into the steel from the surface, and then dissociates, causing local disintegration of the metal’s crystal lattice. This can lead to the development of hydrogen-induced microcracks, which, under the high pressure of the gas pipeline and continued hydrogen exposure, may result in hydrogen cracking. As mentioned in [9], storing hydrogen in underground gas storage (UGS) facilities, along with natural gas, might induce a variety of effects, including steel hydrogenation, thereby intensifying the internal corrosion of steel structures and pipelines. The potential effects of hydrogen on steel during transportation and storage are not thoroughly understood and are vital subjects for future research.
Therefore, it is imperative to explore the trends, progression, and mechanisms of corrosion damage, along with the protective strategies employed at gas processing facilities to counteract HSC and steel hydrogenation. The corrosive effects of H2S and hydrogen ions, generated during the cathodic process, impact steel not only in the liquid phase but also in the vapor phase. This dual-phase impact requires careful consideration when evaluating the corrosive risks of an environment and choosing appropriate protective measures.
Materials and methods
The following types of carbon and low-alloy steels were selected for testing: 09G2S, St20, S–75, X42SS, and 30KhMA. These steels are commonly utilized in different components and segments of wellhead equipment and pipeline systems within gas field operations.
To assess their performance under HSC conditions and to collect corrosion products for subsequent analysis, tests were carried out in controlled environments using autoclaves. These simulated conditions mimicked the effects of mineral content in the environment and the partial pressures (P, MPa) of СО2 and/or H2S, allowing for the replication of the corrosion rates observed in actual field conditions. The autoclave tests were conducted for 120 h at temperatures (T) of 30 or 90 °C. Aqueous environment with mineralization level of 100 (MB1) and 200 g/l (MB2), with the addition of 0.25 g/l of CH3COOH, were used. A 0.5 % NaCl solution was used for the tests in the presence of hydrogen. The total corrosion rate (K, mm/yr) was determined based on the weight loss of the samples from the test results. The effect of hydrogenation was evaluated by the steel’s ability to retain plasticity before and after exposure to the corrosive environment, measured by the number of kinks in wire samples (SV08A steel) before destruction according to GOST 1579 – 93 [10]. The decrease in plasticity (Pl, %) was determined by the number of steel wire kinks after autoclave tests compared to the initial value.
Metallographic analysis of the steels included determining the microstructure according to GOST 8233 [11]. A Zeiss Axio.Vert A1 inverted metallurgical microscope with the Thixomet image analyzer was used for metallographic studies at magnifications of 100 – 1000 times.
The technique for analyzing the phase composition of corrosion products by X-ray diffraction (XRD) method, based on registering the dependence of X-ray reflection intensity (reflections) by the crystal lattices of compounds on the diffraction angle, was described earlier in paper [2], with subsequent interpretation of the diffraction pattern.
Results and discussion
The microstructure of steels significantly influences in the development of corrosion defects and hydrogen permeation into the metal in scenarios involving HSC. According to [12], the highest amount of hydrogen diffuses into the sample of heat-treated carbon steel API X65, a commonly used foreign pipe steel grades, that exhibits a microstructure with an increased proportion of pearlite and a reduced amount of ferrite, akin to the types studied by the authors. This specific microstructural composition leads to diminished steel strength and plasticity. Notably, within the pearlite phase – characterized by its lamellar structure of alternating cementite and ferrite – gaps between the lamellae act as channels that facilitate the diffusion and accumulation of hydrogen. The occurrence of microcracking as a result of hydrogen sulfide embrittlement within the steel’s crystalline structure may limit further permeation of hydrogen into steel. This phenomenon could partly account for the non-uniform distribution of hydrogen across the steel’s thickness, with the highest concentrations typically found in the surface layer. The presence of corrosion-active non-metallic inclusions (CANMIs) in the steel can also contribute to the deterioration of steel strength properties in instances of internal corrosion. Manganese compounds such as MnS, often found in combination with aluminum inclusions in steel, serve as notable examples of CANMIs that function as hydrogen “traps” [13]. The interface between these inclusions and the steel matrix acts as a repository for hydrogen atoms, providing a locale for these atoms to recombine into molecular H2 .
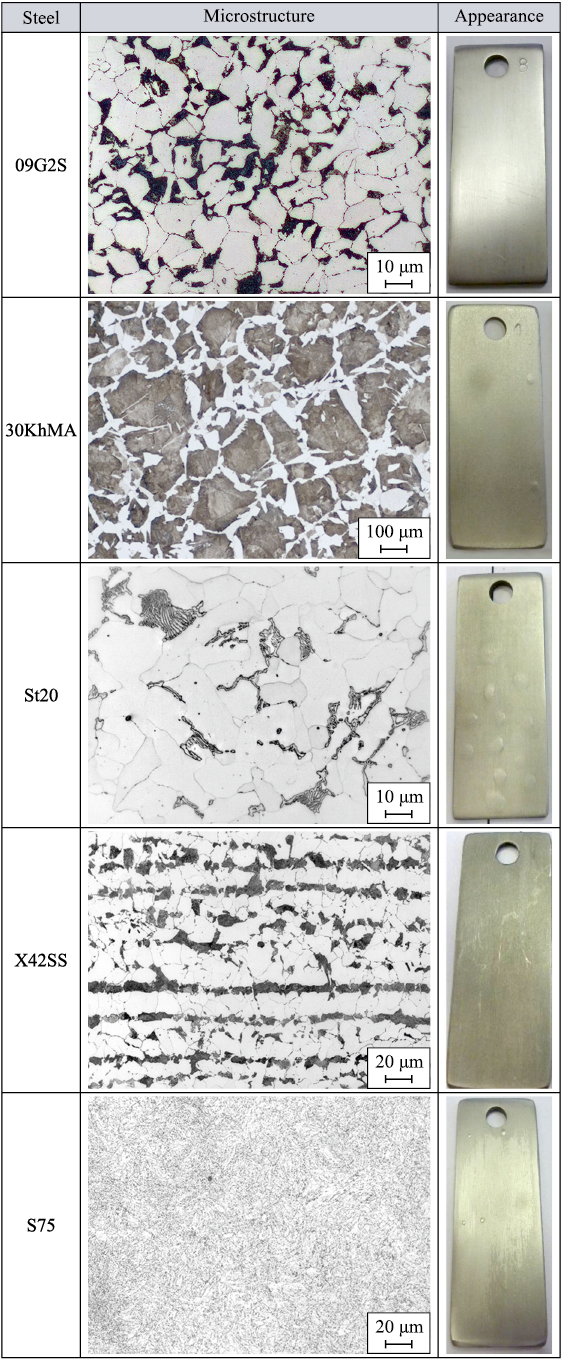
This interaction highlights a critical mechanism through which the microstructure of steel, influenced by the presence of such inclusions, can impact the overall resilience and integrity of the material in corrosive environments, particularly those encountered in gas fields (Figure).
Microstructure and appearance of various structural steels |
The microstructure of the samples is as follows:
– S75 steel exhibits finely dispersed secondary sorbite that has retained martensitic orientation;
– X42SS is a ferritic-perlitic steel;
– St20 is a ferritic-perlitic steel with non-uniform distribution of lamellar pearlite;
– 30KhМА is a coarse-grained ferritic-perlitic steel where ferrite forms a network at the boundaries of primary austenitic grains, and ferrite needles grow from the ferrite network into perlite;
– 09G2S is a fine-grained ferritic-perlitic steel.
Hydrogen permeation has visibly altered the appearance of the steel samples, as evidenced by the formation of blisters of varying sizes on the surface of most steels under examination, with 09G2S steel presenting the smallest blisters. The surface of the 30KhMA steel is extensively marked with small cracks, and sporadic blistering is also evident. These blisters, when subjected to critical hydrogen pressure levels, rupture and lead to crack formation.
The inherent stresses in the steel’s crystal structure, along with the pre-existing microstructural characteristics, are implicated in the initiation and expansion of microcracks [14]. According to the findings in [15], the occurrence of blisters on 13KhFA and 05KhGB steels is related to the presence of complex corrosion-active non-metallic inclusions within the metal, which also predispose these steels to cracking.
Subsequent tests were conducted on St20 steel, which exhibited the highest blister count [2], and 09G2S steel, which is predominantly used in gas infrastructure. Both St20 and 09G2S steels possess a ferritic-perlitic microstructure with a non-uniform distribution of these phases, contributing to their heterogeneous nature. This heterogeneity compromises their resistance to both corrosion and hydrogenation.
Table 1. Conditions and results of simulation tests in H2S-containing environments
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 1 reveals that the K value of the samples made from St20 and 09G2S steels after the tests in and above the MB2 aqueous solution ranges from 0.319 to 0.569 mm/yr (for the aqueous phase) and from 0.156 to 0.227 mm/yr (for the vapor phase). In a different MB1 environment, the K value is higher: 0.8 and 0.52 – 0.55 mm/yr in and above the aqueous electrolyte, respectively. The probable reason is that solubility of corrosive H2S in the higher mineralized MB2 solution is limited. High mineralization can result in lower evaporation and condensation of such water required for HSC to propagate on the samples in the vapor phase. However, despite this, localized corrosion defects are observed on the steel surface of the sample tested above the MB1 solution (Table 2). Tests conducted by the authors under the conditions of forced moisture condensation on carbon steels with H2S purging showed [16] that in the humidified vapor phase, the local K value, calculated by the depth of pittings formed, reached 1.232 – 1.366 mm/yr.
Table 2. Characteristics of the precipitate and crystal structure
| ||||||||||||||||||||||||||||||
The microstructural characteristics of steel, particularly the inclusion of CANMIs such as manganese sulfides, are pivotal in the development of pitting lesions in HSC scenarios [17]. These CANMIs, due to their dissimilarity with the steel matrix, act as precursors for the formation of localized corrosion defects. This typically occurs either through the dissolution of the CANMIs themselves or the steel immediately surrounding them. One of the primary reasons behind the corrosive influence of CANMIs is their heterogeneity, which induces elevated stress levels in the adjacent steel matrix. This stress is a result of the mismatched thermal expansion coefficients between the CANMIs and the steel matrix during the steel’s production, which involves cycles of heating and subsequent cooling [18]. After the steel cools the region with increased tensile stresses may form around the CANMIs, accelerating internal corrosion.
The effect of steel hydrogenation was evaluated by the reduction of its plastic properties, subsequently resulting in hydrogen embrittlement, and deterioration of metal strength characteristics. As shown in Table 1, the Pl value after tests at 30 °C in both environments with H2S ranges from 60 to 74 % for the aqueous phase and from 35 to 66 % for the vapor phase. Traces of blistering are observed on the surface of the samples after exposure to the aggressive environment (Table 2). It should be noted that in both phases for the MB2 environment, when the test temperature is increased to 90 °C, the K value decreases by 33 %, and Pl drops by 45 to 50 % (Table 1). Hydrogenation may slightly decrease as the temperature rises due to higher rates of conjugated electrochemical reactions: the cathodic process of hydrogen atom molization on the steel surface is intensified, reducing their ability to permeate inside the metal, as observed by the authors [19]. Another reason why hydrogen permeation and HSC are limited may be the fact that at elevated temperatures, densely packed and evenly distributed corrosion products form faster, acting as a barrier that creates obstacles preventing corrosion components from permeating to the steel surface.
The assessment of the samples’ appearance after simulation tests and XRD analysis revealed (Table 2) that corrosion products in the vapor and water phases have different thicknesses and phase compositions. Precipitations in the vapor phase form when the water film is thin. It is suggested that in the vapor phase, only 40 % of the compounds of the corrosion products have a crystalline structure. The majority of them (60 %) did not have enough time to form and remained as looser and unconsolidated X-ray amorphous compounds. In the aqueous phase, the sediment film completely crystallized, becoming thicker and denser.
The results of XRD analysis (Table 2) indicate that in both phases, the main corrosion product is iron sulfide, represented by its two crystal forms: tetragonal FeS (mackinawite) and cubic FeS. The presence of cubic FeS distinguishes these results from the previously obtained data [2]. The results presented in this paper show significant differences, as CH3COOH was added to the aqueous environments. Such acidification of the environment alters the composition of corrosion products and leads to the formation of cubic FeS in addition to the tetragonal form. As a volatile compound, CH3COOH evaporates along with H2S and contributes to steel fracture in the vapor phase. Additionally, in this phase, cubic FeS precipitates are also formed (Table 2). The formation of deposits with different crystal structures and different faces of FeS will make the film less cohesive and monolithic, thus reducing its barrier (protective) functionality.
The XRD method is based on the phenomenon of X-ray diffraction on a three-dimensional crystal lattice. In this case, X-rays scattering by crystals correlates with the arrangement of atoms in the crystal. Therefore, each crystalline substance is characterized by a set of peaks in the diffraction pattern, where the position of their reflections is constant, and the relative intensity depends on the substance’s content in the mixture. The main factors causing changes in the width of the reflections are the structural features of phases (such as the size of crystallites – coherent scattering region (CSR)) or the structural features of the crystal lattice. The EXD data facilitates the determination of the crystallite size distribution (CSD) within a polycrystal or identifies the minimum particle size maintaining an accurate crystal structure. The methodology for calculating the CSD relies on the analysis and application of the broadening of diffraction lines, represented by β, employing the Selyakov-Scherrer equation [20]. This technique underpins the evaluation of the CSD for FeS (T) compound particles. As illustrated in Table 2, the experimentally measured β values for the samples (post-exposure to aqueous and vapor phases) exhibit variations of different magnitudes from the standard value (β = 0.1697°), which is typical for the 001 peak position of mackinawite. These discrepancies suggest the occurrence of lattice defects (such as microstresses, isomorphism, etc.) in the samples. Additionally, it was observed that mackinawite crystals formed in the aqueous phase have a significantly larger CSD, up to eight times that of corrosion products formed in the vapor phase. As a result, the FeS film produced in the vapor phase is thinner and possesses inferior protective qualities compared to its aqueous phase counterpart. The findings imply that crystal growth is more rapid in the aqueous phase than in the vapor phase. Moreover, as per [21], a temperature rise from 25 to 80 °C also contributes to an enlargement of crystal compound facets, particularly noticeable in the context of HSC in aqueous environments.
In HSC, corrosion products start to form immediately upon exposure to an aqueous environment containing hydrogen sulfide, a phenomenon observable through visual inspection during experiments. The composition and thickness of these corrosion products are influenced by the operational conditions. Numerous studies under HSC conditions have corroborated that the nature and consistency of the deposits significantly impact corrosion processes [22] and the capability of hydrogen to permeate into steel in aqueous environments [21].
Research documented in [23] indicates that the rate of hydrogen permeation during HSC is influenced by Р\(_{{{\rm{H}}_{\rm{2}}}{\rm{S}}}\), which affects the morphology and structure of the corrosion films formed. Investigations on API X65 pipeline steel in environments containing H2S have shown that the incidence of hydrogen cracking is contingent on both Р\(_{{{\rm{H}}_{\rm{2}}}{\rm{S}}}\) and the pH level [24]. As Р\(_{{{\rm{H}}_{\rm{2}}}{\rm{S}}}\) increased from 0.001 to 0.1 MPa, there’s a predictable rise in the number of visible microcracks on the steel surface. However, the pH level exerts a more significant influence on hydrogen permeation; in acidic conditions (pH = 3.5), permeation rates surpass those in relatively neutral conditions (pH ranging from 5.5 to 6.5). For example, at Р\(_{{{\rm{H}}_{\rm{2}}}{\rm{S}}}\) = 0.1 MPa and pH = 5.5, the amount of hydrogen absorbed by the steel decreased and, consequently, the effect of hydrogen cracking on the steel surface decreased. The authors attribute to the formation of denser and more uniformly distributed corrosion products (FeS) at the highest Р\(_{{{\rm{H}}_{\rm{2}}}{\rm{S}}}\) = 0.1 MPa), which impede hydrogen permeation. Additionally, the formation of mackinawite may decelerate the anodic process of HSC, thereby slowing down the associated electrochemical reaction of hydrogen formation. Another study [25] has noted a reduction in hydrogen diffusion over time, resulting from the development of denser corrosion layers that obstruct the anodic-cathodic process of HSC on pipeline steel.
Visual examination of samples post-exposure to the MB2 environment (Table 3) reveals the formation of blisters of varying sizes on St20 steel in the absence of H2S protection measures, like corrosion inhibitors, under identical conditions (11). This variability in blister formation is likely due to the unique microstructural features of St20 steel, which exhibits a notably uneven distribution of the pearlite phase. Within the ferritic matrix, pearlite manifests as both discrete particles and sizeable clusters. Such microstructural diversity creates varying sizes of hydrogen “traps,” thereby affecting the morphology of the resulting blisters.
Table 3. Appearance of the samples after testing in the MB1 environment
| |||||||||||||||||||||||||||||||||
Recent developments in the pipeline transportation of hydrogen, including blends of hydrogen with methane, have seen significant interest. According to a review in paper [8], studies from abroad conducted over the past decade or so reveal that existing data on the operation of main gas pipelines for transporting methane-hydrogen mixtures are not comprehensive. This is primarily because the fundamental parameters, such as strength and steel composition, differ significantly, complicating the identification of consistent patterns regarding hydrogen’s impact on steel. Furthermore, the operational performance of these pipelines has been deemed inadequate for future needs. The hydrogen content in these methane-hydrogen mixtures varies from a minimal 2 – 3 % to a maximum of 20 %, with transportation distances spanning several tens of kilometers, and the total pressure not surpassing 1 MPa. The authors of [8] suggest that the observed low performance is attributed to the utilization of older main gas pipelines, which were subject to operational limitations. This has led to varied data regarding the safe hydrogen content or the critical Р\(_{{{\rm{H}}_{\rm{2}}}}\) value in the hydrogen-methane mixture. However, there is a consensus among researchers [26 – 27] that a higher H2 content could exacerbate the wear on pipeline steel, adversely affecting its plasticity, ductility, and resistance to crack propagation.
Furthermore, it was previously noted [28] that СО2 is commonly encountered at oil and gas facilities and may be transported alongside H2 through gas pipelines. This scenario becomes particularly relevant when free moisture is present, such as during condensation [4]. In such cases, the chemical interactions involving H2 (with СО2 present in the natural gas) can act as an additional source of reagents that initiate internal corrosion, further complicating the challenges associated with transporting these gases. The research cited in [9] identifies lower carboxylic acids as intermediate products of chemical interactions that acidify the environment, posing a corrosive threat to steel and potentially enhancing the cathodic process of steel hydrogenation [1]. During the transport of methane-hydrogen mixtures, СО2 can be present as an impurity, with up to 2.4 % allowed in natural gas through main gas pipelines. Studies [29] have demonstrated that the presence of both H2 and СО2 simultaneously increases the rate of fatigue crack growth more than in an environment containing only hydrogen. This suggests a synergistic effect of СО2 on hydrogen embrittlement, yet the mechanism behind this interaction remains poorly understood and warrants further investigation.
Subsequent experiments were conducted to examine wire plasticity in an H2 environment akin to the hydrogen sulfide conditions previously discussed. It was found (Table 4) that in an aqueous environment (0.5 % NaCl solution), steel plasticity is on average 50 – 60 % lower when 50 – 60 % lower when H2 and СО2 are present during together. However, these tests did not show any significant effect of СО2 on hydrogen permeation (Table 4), indicating that more research is needed in this area.
Table 4. Results of the studies in 0.5 % NaCl solution
| ||||||||||||||||||||||||||||||||||||||||||
Interestingly, in the vapor phase of H2 and СО2 (without contact with water), no decrease in steel plasticity was observed, similar to conditions where the water is saturated with СО2 only. This suggests that the tests did not reveal any impact of hydrogen on steel’s plastic properties in the vapor phase (Table 4) possibly due to the short duration of the tests (120 h). In contrast, hydrogenation and subsequent hydrogen embrittlement of steel occur much more rapidly in an H2S environment (Table 1) than with H2 alone. According to [30], the increase in hydrogen cracking in an H2 environment is linked to the greater amount of hydrogen absorbed by the steel, with the time required for hydrogen to permeate into steel’s dislocations being a critical factor. Thus, the duration of hydrogen exposure is a significant aspect to consider in future experimental designs.
The paper [31] further elucidates the impact of hydrogen on gas main pipelines, noting a reduction in plasticity and crack resistance of pipe steels by 20 – 60 %, and a significantly higher crack propagation rate. Simultaneously, the risk of hydrogen embrittlement increases as the hydrogen concentration in the hydrogen-methane mixture rises, leading to a corresponding increase in Р\(_{{{\rm{H}}_{\rm{2}}}}\). Research cited in [32] identifies a critical Р\(_{{{\rm{H}}_{\rm{2}}}}\) range of 0.05 to 0.1 MPa for austenitic stainless steel type 304SS, beyond which the material begins to exhibit embrittlement at room temperature in an inert gas environment containing hydrogen.
Studies [29; 31] have shown that hydrogen presence shifts the dominant failure mode of steel from ductile to brittle, thereby altering its mechanical properties. It’s highlighted in [31] that both H2 and H2S environments can reduce the plasticity of steel and intensify hydrogen embrittlement. Specifically, steel samples with a streaked ferritic-perlitic structure exhibited lower resistance to a hydrogen-methane mixture than those with a more uniform ferrite-bainite structure. Further findings [33] indicate that hydrogen crack initiation in X42 pipeline steel, exposed to a hydrogen (Р\(_{{{\rm{H}}_{\rm{2}}}}\) ranging from 0.5 to 10 MPa) and natural gas mixture, tends to occur at the ferrite/perlite interface. This interface is a critical site for hydrogen diffusion, especially under local stress or strain along grain boundaries.
Mitigation strategies against the detrimental effects of hydrogen include modifying the steel’s microstructure to reduce factors that contribute to H2 accumulation and employing corrosion inhibitors. Among the primary defenses against hydrogen sulfide cracking (HSC) is the use of corrosion inhibitors.
One of the main means of protection against HSC is the use of corrosion inhibitors. However, it should be noted that under such conditions the impact of hydrogen sulfide embrittlement on steel pipes and equipment seriously complicates the selection of potential corrosion inhibitors, in addition to aggravating corrosion [34 – 35].
Technologies for administering corrosion inhibitors encompass injecting them into the aqueous environment or forming an inhibitor coating to safeguard steel in the vapor phase. The conducted experiments reveal (Table 3) that the vapor-phase inhibitor coating can effectively protect the steel using the specified corrosion inhibitor (corrosion rate below 0.1 mm/yr), but it fails to entirely prevent defect formation on the steel’s surface. Meanwhile, steel hydrogenation was found to be minimal: hydrogen uptake was 4.1 % at 30 °C and increased to 21.7 % at 90 °C. In contrast, inhibitor coatings from other chemicals not discussed in this document provided even less protection against hydrogen sulfide cracking (HSC) (corrosion rates ranging from 0.187 to 0.303 mm/yr) and hydrogenation (hydrogen uptake between 34 and 64 %), resulting in numerous pits on the steel surface.
When the most effective inhibitor was introduced into the aqueous environment, overall corrosion damage was minimal (K = 0.013 – 0.038 mm/yr), and steel retained high plasticity (Pl = 8.8 – 12.9 %) (Table 3). However, an initial inhibitor concentration of 100 mg/l proved to be insufficient at 90 °C, as localized damage occurred on the steel surface. Increasing the inhibitor concentration in the aqueous phase by 2 to 3 times successfully prevented localized corrosion on the steel surface at elevated temperatures during the test (Table 3).
The occurrence of increased pitting corrosion might be attributed to either an inadequate concentration of the corrosion inhibitor, preventing the formation of a uniform protective layer on the steel, or a low adsorption rate on the metal surface compared to that of reactive iron sulfide (FeS). According to the study referenced in paper [34], the competitive adsorption between iron sulfide and the corrosion inhibitor on the steel surface might undermine the effectiveness of the inhibitor film.
Additionally, employing corrosion inhibitors in an aqueous environment saturated with Н2 and СО2 , was also effective in preserving the plasticity of steel (Table 4).
Research aimed at improving steel properties and enhancing their resistance to hydrogen permeation is also ongoing. The paper [36] gives a positive example when the homogeneous ferrite-bainite microstructure of steel with enhanced resistance to hydrogen cracking is formed. The regulation of the thermal modes of steel quenching and tempering enables obtaining the metal structure with such levels of microstresses and dislocation density that resistance to cracking in the H2S environment can be increased [37]. The studies [12] proved that the ratio of ferrite and pearlitic components can be changed by increasing the temperature from 850 to 1150 °C as API X65 pipe steel is formed. The content, stability, and sizes of the faces of ferrite grain increase, while simultaneously, the pearlite content in the steel decreases from 15 to 2 %. As the proportion of ferrite in the steel increases, there is a notable reduction in both cathodic and anodic reaction rates within H2S environments, alongside a decrease in the accumulation of hydrogen within “traps”. To bolster the resistance of structural steels to cracking when exposed to H2S environments, a comprehensive strategy is employed. This strategy involves the modification of steel through the addition of calcium and rare-earth elements, in conjunction with processes such as quenching followed by tempering. These modifications are aimed at preventing the formation of CANMIs, like iron sulfides, within the steel matrix. By inhibiting the development of these inclusions, the approach effectively reduces the emergence of microstresses in their vicinity. Consequently, this leads to a decrease in microcrack formation, thereby diminishing the risk of hydrogen-induced cracking in the steel.
Conclusions
Research analysis and literature review reveal that steel’s plasticity is adversely affected by hydrogenation in hydrogen sulfide environments, such as those encountered in gas fields, as well as in the presence of molecular hydrogen, whether alone or combined with other gases. The conditions prevalent in H2S-containing gas fields pose a significant aggressive influence on the cracking of pipelines and equipment constructed from carbon and low-alloy steels. This susceptibility to hydrogenation is manifested through the development of cracks and blisters on the surfaces of various steels. The propensity for hydrogen to permeate and be stored in steel is heightened and influenced by microstructural inhomogeneities, including CANMIs, as well as the distribution and dimensions of ferrite-perlite components within the steel. It has been noted that the corrosion film that forms on steel in vapor phase environments is significantly thinner than that formed in aqueous conditions. In environments that are acidified by acetic acid, the cubic form of iron sulfide emerges alongside mackinawite (tetragonal FeS, the primary corrosion product), resulting in a less compact film that diminishes its protective capabilities. The physical characteristics of these deposits, such as their density, compactness, presence of pores, and thickness, play a crucial role in determining their effectiveness as barriers against both HSC and the permeation of hydrogen into the steel. Employing corrosion inhibitors has been shown to effectively preserve the plasticity of steel at adequate levels in environments containing H2 and H2S, including scenarios where СО2 is also present.
References
1. Vagapov R.K. Corrosion destruction of steel equipment and pipelines at gas field facilities in the presence of aggressive components. Steel in Translation. 2023;53(1):5–10. https://doi.org/10.3103/S0967091223010138
2. Kantyukov R.R., Zapevalov D.N., Vagapov R.K. Assessment of the effect of operating conditions on the resistance of steels used in H2S-containing environments at hydrocarbon production facilities. Metallurgist. 2022;65:1369–1380. https://doi.org/10.1007/s11015-022-01284-4
3. Ghosha G., Rostron P., Garg R., Panday A. Hydrogen induced cracking of pipeline and pressure vessel steels: A review. Engineering Fracture Mechanics. 2018;199:609–618. https://doi.org/10.1016/j.engfracmech.2018.06.018
4. Vagapov R.K., Kantyukov R.R., Zapevalov D.N. Investigation of the corrosiveness of moisture condensation conditions at gas production facilities in the presence of СО2. International Journal of Corrosion and Scale Inhibition. 2021;10(3):994–1010. https://doi.org/10.17675/2305-6894-2021-10-3-11
5. Zhirnov R.A., Derbenev V.A., Lyugai A.D., Polozkov K.A., Semikolenov T.G., Nikitin V.V., Dymochkina M.G. Prospects for acid gas re-injection into the formation to improve the efficiency of field development (on the example of the Astrakhan GCF). Nauka i tekhnika v gazovoi promyshlennosti. 2020;(1(81)):32–39. (In Russ.).
6. Aksyutin O.E., Ishkov A.G., Romanov K.V., Teterevlev R.V. Methane-hydrogen energy for low-emission development. Gazovaya promyshlennost`. 2018;(11(777)):120–125. (In Russ.).
7. Messaoudani Z.L., Rigas F., Hamid M.D.B., Hassan C.R.C. Hazards, safety and knowledge gaps on hydrogen transmission via natural gas grid: A critical review. International Journal of Hydrogen Energy. 2016;41(39):17511–17525. http://dx.doi.org/10.1016/j.ijhydene.2016.07.171
8. Wu X., Zhang H., Yang M., Jia W., Qui Y., Lan L. From the perspective of new technology of blending hydrogen into natural gas pipelines transmission: Mechanism, experimental study, and suggestions for further work of hydrogen embrittlement in high-strength pipeline steels. International Journal of Hydrogen Energy. 2022;47(12):8071–8090. https://doi.org/10.1016/j.ijhydene.2021.12.108
9. Kireeva Т.А., Berestovskaya Yu.Yu. Microbiological shifts in hydrogen-rich gases under storage. Gazovaya promyshlennost`. 2012;(5(684)):51–54. (In Russ.).
10. GOST 1579-93 Wire. Bend test method. (In Russ.).
11. GOST 8233-56 Steel. Microstructure standards. (In Russ.).
12. Mousavi Anijdan S.H., Arab Gh., Sabzi M., Sadeghi M., Eivani A.R., Jafarian H.R. Sensitivity to hydrogen induced cracking, and corrosion performance of an API X65 pipeline steel in H2S containing environment: influence of heat treatment and its subsequent microstructural changes. Journal of Materials Research and Technology. 2021;15:1–16. https://doi.org/10.1016/j.jmrt.2021.07.118
13. Quispe-Avilés J.M., Pereira Fiori M.A., Hincapie-Ladino D., Prada Ramirez O.M., Gomes de Melo H. Effects of Mn and microalloying composition on corrosion and hydrogen-induced cracking of API 5L X65 steels. Corrosion. 2022;78(8):765–777. https://doi.org/10.5006/3876
14. Huang F., Li X.G., Liu J., Qu Y.M., Du C.W. Effects of alloying elements, microstructure, and inclusions on hydrogen induced cracking of X120 pipeline steel in wet H2S sour environment. Materials and Corrosion. 2012;63(1):59–66. https://doi.org/10.1002/maco.201005649
15. Naumenko V.V., Mursenkov E.S., Kudashov D.V., Udod K.A. Study of reasons for the blistering formation on metal surface after the tests on hydrogen induced cracking and classification of blisterings. Steel in Translation. 2022;52(2):263–269. https://doi.org/10.3103/S0967091222020139
16. Ibatullin K.A., Vagapov R.K. Evaluation of the influence of various factors on the corrosion of steels during moisture condensation under the conditions of transportation of a corrosive gas. Praktika protivokorrozionnoi zaschity. 2022;27(3):31–46. (In Russ.).
17. Amezhnov A.V., Rodionova I.G. Effect of non-metallic inclusion chemical and phase composition on corrosion resistance of carbon and low alloy steels in water media typical for oilfield pipeline operating conditions. Metallurgist. 2019;63:717–726. https://doi.org/10.1007/s11015-019-00881-0
18. Golubtsov V.A., Ryabchikov I.V., Mizin, V.G. Influence of chemically active elements on the hydrogen cracking of pipe steel. Steel in Translation. 2016;46(3):220–223. https://doi.org/10.3103/S0967091216030037
19. Talukdar A., Rajaraman P.V. Effect of acetic acid in CO2-H2S corrosion of carbon steel at elevated temperature. Materials Today: Proceedings. 2022;57(4):1842–1845. https://doi.org/10.1016/j.matpr.2022.01.036
20. Mikhalkina O.G., Fedorov P.P., Andreev P.O. Obtaining compounds of rare earth elements using sulfides. Khimicheskaya Tekhnologiya. 2011;12(12):706–710. (In Russ.).
21. Zhou C., Chen X., Wang Z., Zheng S., Li X., Zhang S. Effects of environmental conditions on hydrogen permeation of X52 pipeline steel exposed to high H2S-containing solutions. Corrosion Science. 2014;89:30–37. https://doi.org/10.1016/j.corsci.2014.07.061
22. Liu Z., Wang Y., Hai Y., Qiao Y., Zheng C., Wang D., Shi X., Lu H., Liu C. Corrosion behavior of low alloy steel used for new pipeline exposed to H2S-saturated solution. International Journal of Hydrogen Energy. 2022;47(77):33000–33013. https://doi.org/10.1016/j.ijhydene.2022.07.203
23. Alanazi N.M., Al-Enezi A.A. The effect of the partial pressure of H2S and CO2 on the permeation of hydrogen in carbon steel by using pressure buildup techniques. Corrosion. 2019;75(10):1207–1215. https://doi.org/10.5006/3128
24. Kittel J., Smanio V., Fregonese M., Garnier L., Lefebvre X. Hydrogen induced cracking (HIC) testing of low alloy steel in sour environment: Impact of time of exposure on the extent of damage. Corrosion Science. 2010;52(4):1386–1392. http://dx.doi.org/10.1016/j.corsci.2009.11.044
25. Huang B., Peng H., Chen X., Gong C., Li J. Study on the impact toughness and diffusible hydrogen of G105 drill pipe steel in wet H2S environment. Corrosion Engineering, Science and Technology. 2017;52(6):453–458. https://doi.org/10.1080/1478422X.2017.1329247
26. Laureys A., Depraetere R., Cauwels M., Depover T., Hertelé S., Verbeken K. Use of existing steel pipeline infrastructure for gaseous hydrogen storage and transport: A review of factors affecting hydrogen induced degradation. Journal of Natural Gas Science and Engineering. 2022;101:104534. https://doi.org/10.1016/j.jngse.2022.104534
27. Zhou D., Li T., Huang D., Wu Y., Huang Z., Xiao W., Wang Q., Wang X. The experiment study to assess the impact of hydrogen blended natural gas on the tensile properties and damage mechanism of X80 pipeline steel. International Journal of Hydrogen Energy. 2017;42(10):7407–7412. https://doi.org/10.1016/j.ijhydene.2020.11.267
28. Kantyukov R.R., Zapevalov D.N., Vagapov R.K. Media corrosiveness and materials resistance at presence of aggressive carbon dioxide. Izvestiya. Ferrous Metallurgy. 2021;64(11):793–801. (In Russ.). https://doi.org/10.17073/0368-0797-2021-11-793-801
29. Shang J., Chen W., Zheng J., Hua Z., Zhang L., Zhou C., Gu C. Enhanced hydrogen embrittlement of low-carbon steel to natural gas/hydrogen mixtures. Scripta Materialia. 2020;189:67–71. https://doi.org/10.1016/j.scriptamat.2020.08.011
30. Song E.J., Baek S.-W., Nahm S.H., Baek U.B. Notched-tensile properties under high-pressure gaseous hydrogen: Comparison of pipeline steel X70 and austenitic stainless type 304L, 316L steels. International Journal of Hydrogen Energy. 2017;42(12):8075–8082. http://dx.doi.org/10.1016/j.ijhydene.2016.12.069
31. Nastich S.Yu., Lopatkin V.A., Arabei A.B., Egorov V.A., Popkov A.S. Changes in the mechanical properties of metal pipes of main gas pipelines under the influence of hydrogen gas at high pressure. In: Gas Transportation Systems: Present and Future (GTS-2023): Abstracts of Reports of the IX International Scientific and Technical Conference. Kazan, 03–07 April 2023. Kazan: Gazprom VNIIGAZ; 2023:27. (In Russ.).
32. Koide K., Minami T., Anraku T., Iwase A., Inoue H. Effect of hydrogen partial pressure on the hydrogen embrittlement susceptibility of type 304 stainless steel in high pressure H2/Ar mixed gas. ISIJ International. 2015;55(11):2477–2482. http://dx.doi.org/10.2355/isijinternational.ISIJINT-2015-232
33. Nguyen T.T., Park J.S., Nahm S.H., Baek U.B. Evaluation of hydrogen related degradation of API X42 pipeline under hydrogen/natural gas mixture conditions using small punch test. Theoretical and Applied Fracture Mechanics. 2021;113:102961. https://doi.org/10.1016/j.tafmec.2021.102961
34. Pessu F., Barker R., Chang F., Chen T., Neville A. Iron sulphide formation and interaction with corrosion inhibitor in H2S-containing environments. Journal of Petroleum Science and Engineering. 2021;207:109152. https://doi.org/10.1016/j.petrol.2021.109152
35. Tsygankova L.E., Uryadnikov A.A., Abramov A.E., Semenyuk T.V. Inhibiting formulations against hydrogen sulfide corrosion of carbon steel. International Journal of Corrosion and Scale Inhibition. 2022;11(1):102–110. https://doi.org/10.17675/2305-6894-2021-11-1-5
36. Matrosov Yu.I., Kholodnyi A.A., Matrosov M.Yu., Popov E.S., Konovalov G.N., Sosin S.V. Effect of accelerated cooling parameters on microstructure and hydrogen cracking resistance of low-alloy pipe steels. Metallurgist. 2015;59:60–68. https://doi.org/10.1007/s11015-015-0061-1
37. Naumenko V.V., Muntin A.V., Baranova O.A., Smetanin K.S. Resistance to hydrogen cracking of rolled structural steel after heat processing. Steel in Translation. 2021;51(3): 211–216. https://doi.org/10.3103/S0967091221030086
About the Authors
R. R. KantyukovRussian Federation
Rafael’ R. Kantyukov, Cand. Sci. (Eng.), Deputy General Director for Research
Razvilka Village, Moscow Region 142717, Russian Federation
D. N. Zapevalov
Russian Federation
Dmitry N. Zapevalov, Cand. Sci. (Eng.), Head of the Corporate Scientific and Technical Center for Corrosion Monitoring and Protection
Razvilka Village, Moscow Region 142717, Russian Federation
R. K. Vagapov
Russian Federation
Ruslan K. Vagapov, Dr. Sci. (Eng.), Cand. Sci. (Chem.), Head of the Laboratory of Atmospheric and Internal Corrosion Protection
Razvilka Village, Moscow Region 142717, Russian Federation
Review
For citations:
Kantyukov R.R., Zapevalov D.N., Vagapov R.K. Effect of hydrogen on steels in hydrogen sulfide-containing and other environments at gas facilities. Izvestiya. Ferrous Metallurgy. 2024;67(1):53-64. https://doi.org/10.17073/0368-0797-2024-1-53-64