Scroll to:
Production of refining alumina-containing fluxes by sintering from technogenic raw materials
https://doi.org/10.17073/0368-0797-2023-6-760-767
Abstract
Modern Russian steelmaking plants use predominantly alumina-containing materials for liquefying lime in a ladle-furnace unit, which replaced fluorspar. Alumina-containing materials currently available on the market cannot be used directly in steelmaking without preliminary preparation (refining, heat treatment or briquetting), or are simply unsuitable for ladle processing of steel. This work describes laboratory studies on the production of refining alumina-containing fluxes by sintering in units such as machines for pellets firing or producing agglomerate (in the temperature range of 1200 – 1500 °C) from clean metallurgical waste (fine dust from the production of alumina and burnt lime), meeting the requirements of steelmaking plants by chemical composition and mechanical properties. A comparison was made of sintering technological schemes with the introduction of hydrated lime and a mixture of hydrated lime and calcium carbonate in a 1:1 ratio as a source of CaO. We determined that the maximum permissible CaO content in sintered briquettes when using a mixture of hydrated lime and calcium carbonate in the charge, which does not lead to hydration destruction in air, is in the range of 2.3 - 3.6 %, depending on the holding temperature. The maximum permissible content of Al2O3 in sintered briquettes when using hydrated lime in the charge, which does not lead to hydration destruction in air, is in the range of 9.5 – 31.7 %, depending on the holding temperature. In existing fuel units it is possible to obtain fluxes by sintering only when using hydrated lime as a source of CaO, because adding calcium carbonate to the charge (9 – 22 %) requires an increase in holding temperature (above 1500 °C) or holding time (more than 25 min).
Keywords
For citations:
Aksenova V.V., Pavlov A.V., Markov G.M. Production of refining alumina-containing fluxes by sintering from technogenic raw materials. Izvestiya. Ferrous Metallurgy. 2023;66(6):760-767. https://doi.org/10.17073/0368-0797-2023-6-760-767
Introduction
The fluxes and slag-forming materials used in metallurgy exert significant influence on production technology, as well as the chemical composition and quality of smelted steel. The quantity of detrimental impurities (such as sulfur, phosphorus, and gases like oxygen, hydrogen, and nitrogen) is contingent upon the type of additives employed, which constitutes a fundamental determinant in achieving high-quality steel.
In the ladle-furnace unit (LFU) utilized for steel processing, lime serves as the primary slag-forming material, boasting a melting point exceeding 2500 °C. To lower the slag’s melting point during extraction, flux is introduced alongside lime to induce liquefaction. In the past, fluorspar was widely employed for slag liquefaction in LFUs; however, its usage has recently been minimized or entirely phased out due to various adverse factors including its short-term efficacy, diminishment of lining resistance in the slag belt zone, and environmental concerns [1 – 3].
Alumina-containing fluxes, surpassing fluorspar in several aspects, emerge as a promising alternative to fluorine-based materials. These alumina-containing materials can be employed either independently or in conjunction with fluorspar, even for steel grades designated as “aluminum-free” [4]. Notably, aluminothermal slags derived from ferrovanadium and aluminothermal chromium production have gained significant traction as alumina-containing fluxes. However, a major drawback lies in their market scarcity due to the limited production of ferroalloys via aluminothermal reduction. Additionally, other available alumina-containing materials often require preliminary treatment (such as refining, heat treatment, or briquetting) before they can be utilized in steelmaking, or they may prove unsuitable for the ladle processing of steel [5 – 7].
When procuring fluxes, steelmaking plants impose criteria concerning both chemical composition and mechanical properties. Fluxes are expected to be supplied in the form of lumps or briquettes, with overall dimensions ranging from 10 to 50 mm. The fine fraction (0 – 5 mm) should not exceed 10 % of the total mass, while moisture content is to be kept below 1 % in summer and up to 6.5 % in winter. Consumers may also specify requirements regarding the strength properties of briquettes/lumps. A summarized outline of the chemical composition requirements for alumina-containing fluxes is presented in Table 1.
Table 1. Consolidated requirements for the chemical composition
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The required quantity of alumina-containing flux for processing in the LFU is determined through the balance equation of aluminum utilization in ladle processing:
Alsec = Alres + Aldeox + Alair + Alsl + Alevap ,
where Alres is residual aluminum, Aldeox is deoxidation aluminum, Alair is air oxygen-oxidized aluminum, Alsl is furnace slag-oxidized aluminum, and Alevap is evaporated aluminum.
A portion of Al2O3 needed for lime liquefaction is generated through the interaction of aluminum with dissolved oxygen in steel, while another portion results from combustion on the slag surface and subsequent deoxidation. The remaining portion is introduced in the form of flux to facilitate free-running slag. Despite its technological efficiency, aluminum is deemed economically impractical due to its high cost as a source of Al2O3 . According to the balance equation, it’s estimated that aluminum consumption is distributed as follows: Alres – 15 %; Aldeox – 18 %; Alair – 38 %; Alsl – 28 %; Alevap – 1 %.
Given the current industrial environmental constraints and stringent steel quality requirements, developing alumina-containing flux production technology in an environmentally sustainable and cost-effective manner emerges as an urgent challenge.
Materials and methods
An essential consideration in flux production via sintering is the meticulous selection of charge materials. Firstly, these materials must not introduce harmful impurities that resist removal during heat treatment and could potentially contaminate the processed steel. Secondly, they should be relatively amenable to briquetting, as sintering technology involves the heat treatment of lump material. Thirdly, availability on the market is crucial.
The recycling of metallurgical waste to yield marketable products has gained significant traction in recent times. Among such wastes is the dust collected from the filters of roasting furnaces. This paper focuses on the processing of dust originating from alumina calcination and limestone roasting furnaces.
Alumina calcination involves the dehydration of aluminum hydroxide at elevated temperatures. Aluminothermal slag, utilized in rotary tube furnaces or fluidized-bed furnaces at temperatures reaching up to 1200 °C, represents the final stage in the Al2O3 production process chain. Inevitably, during the calcination processes in various units, nanoscale dust is generated. Research cited in [8] indicates that the size of dust nanoparticles falls within the range of 50 – 300 nm. Approximately 14 % of the fine alumina dust is carried away from the furnace by flue gas during calcination, which is then directed to multicyclones and electric filters [9]. However, this dust, containing nanoparticles, is unsuitable for use in the classical technology of electrolytic decomposition of Al2O3 due to its hygroscopic nature, leading to excessive hydrogen content in aluminum metal. Nonetheless, this material finds applicability in the iron industry as a source of Al2O3 in steelmaking fluxes.
When selecting a source of CaO for flux production via sintering, one should consider a chain of chemical transformations: CaCO3 → CaO → Ca(OH)2 → CaCO3 .
Fine-dispersed carbonate rocks, burnt, or slaked lime can serve as a source of CaO, each carrying its own set of advantages and disadvantages. Limestone (CaCO3 ) necessitates no preliminary preparation before briquetting. However, during sintering, its decomposition into CaO and CO2 absorbs heat (178 kJ/mol). Burnt lime (CaO), on the other hand, doesn’t undergo significant mass loss during sintering due to the absence of crystal hydrate moisture. Yet, its slaking during briquetting releases heat (65 kJ/mol), which is not conducive to the process. Slaked lime (Ca(OH)2 ) offers several advantages over the aforementioned materials:
– it requires no preliminary preparation and can hydrate in air during storage;
– it facilitates convenient briquetting as it doesn’t generate heat when interacting with water;
– heat absorption during its decomposition into CaO and H2O (65 kJ/mol) during sintering is almost 3 times less than the heat absorbed during the decomposition of CaCO3 .
In industrial settings, burnt lime is typically obtained by calcinating carbonate rocks in shaft or rotary furnaces at temperatures ranging from 1000 to 1250 °C [10]. Analogous to alumina calcination, this process generates micro-sized dust particles (6 – 60 µm), which are typically collected in bag filters or electric filters [11]. While this dust shares a similar composition with the burnt material, its smaller particle size renders it more prone to rapid hydration in the air during storage. Nonetheless, this fine dust can also serve as raw material for the production of sintered alumina-lime fluxes.
An integral aspect of working with dispersed materials involves their preparation for heat treatment. In this study, cold briquetting was employed. Drawing from successful experiments involving porous alumina-containing materials [12], a binder based on polyacrylamide was utilized. This binder exhibits low consumption (0.6 % of the mass of the briquetted raw material) and is completely removed at sintering temperatures.
The production technology of alumina-lime fluxes, designed to withstand hydration and subsequent destruction during sintering, from pure components encompasses the following operations:
– production of briquettes from pure components;
– heating the material to the holding temperature;
– maintaining a constant temperature during holding;
– cooling in ambient air.
In laboratory settings, sintering was conducted in a resistance furnace equipped with a graphite heater. The technologies employed in ore pellet roasting and agglomerate production served as the foundation for flux production. The temperature range for laboratory experiments was selected in accordance with the existing process characteristics of fuel units. Literature data indicates that the maximum roasting temperature for iron ore pellets is around 1400 °C [13 – 17], while for chromite pellets, it ranges from 1400 to 1500 °C [18 – 22]. Hence, the temperature holding interval ranged from 1200 to 1500 °С. During laboratory experiments, the average heating rate was maintained at 20 °C, and the duration of holding at a constant temperature ranged from 15 to 25 min. Following heat treatment, the sintered briquettes were cooled in ambient air, after which their mass and geometric parameters were measured. Subsequently, the briquettes were stored in air, at a temperature of 21 °C and a relative humidity of 50 %, to monitor any changes in mass. Weighing and recording the mass change of the briquettes were conducted once every seven days until the mass stabilized.
Two series of experiments were conducted utilizing different sources of CaO: a mixture of hydrated lime and calcium carbonate in a 1:1 ratio (series 1) and hydrated lime along (series 2). The proportion of Al2O3 in the briquettes before sintering varied from 50 to 80 % in both cases, with increment of 5 %. The chemical composition of the raw materials is provided in Table 2.
Table 2. Chemical composition of raw materials
| |||||||||||||||||||||||||||
Results and discussion
After the briquettes had cooled in air, an external evaluation of their post-sintering state was conducted. Based on their appearance, the heat-treated briquettes were categorized into four conventional groups (Fig. 1)
Fig. 1. Appearance of heat-treated briquettes: |
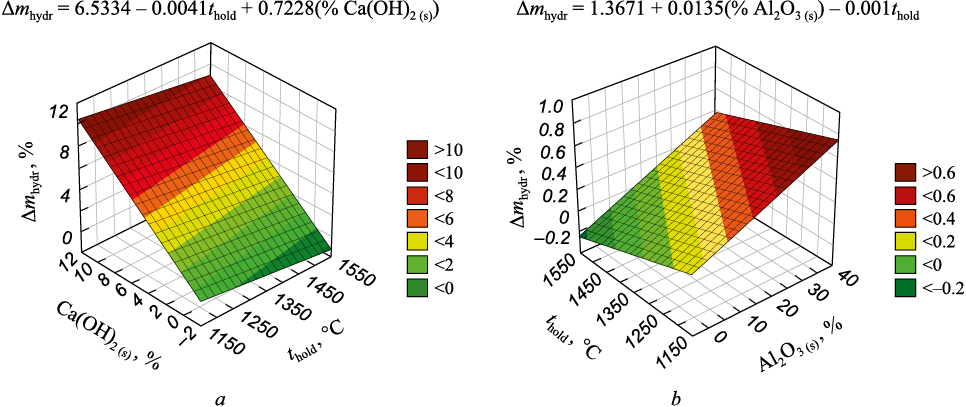
The STATISTICA software package was employed to process the results and identify factors influencing the hydration destruction of sintered briquettes. The dependent variable was the percentage of mass gain during storage in air, while the independent variables included the temperature of sintering (°C), sintering time (min), composition of initial briquettes (%), change in density and volume of briquettes during sintering (%), and phase composition of sintered briquettes after holding in air (%). Fig. 2 illustrates the primary factors influencing the hydration destruction of briquettes for the two series of experiments.
Fig. 2. Influence of holding temperature, Ca(OH)2 and Al2O3 content in sintered materials |
In addition to quantitative evaluation, qualitative assessment of the visual state of briquettes during air storage was conducted. For experiments in series 1, the initial signs of hydration degradation, accompanied by mass gain, were observed as early as the 7\(^{th}\) day of air storage, with the mass gain concluding on the 56\(^{th}\) day of observation. For the experiments in series 2, the first indicators of hydration degradation were observed only on the 28\(^{th}\) day of air storage, with the mass gain ceasing on the 100\(^{th}\) day of observation. The results of X-ray structural analysis of sintered briquettes that remained intact during air storage are presented in Table 3.
Table 3. X-ray structural analysis of sintered briquettes
|
The results of the comprehensive evaluation by regression equations, determining the maximum permissible content of free CaO and Al2O3 in sintered briquettes for two laboratory series, are presented in Table 4.
Table 4. Ultimate content of CaO and Al2O3 in sintered briquettes
| ||||||||||||||||||||||||||||||||||
It has been established that in series 1, where the process of calcium aluminate formation coincides with the decomposition of calcium carbonate and calcium hydroxide, it is necessary to elevate the temperature (>1500 °C) and/or extend the holding time (>25 min) to ensure the completion of all structural transformations. Conversely, series 2 demonstrates that hydration-resistant materials can be produced at relatively low temperatures (starting from 1200 °C) within the specified time interval (15 – 25 min).
Conclusions
The maximum allowable content of CaO in sintered briquettes, which prevents the destruction of the sintered material during air storage (in the case of using a mixture of hydrated lime and calcium carbonate as a source of CaO), falls within the range of 2.3 to 3.6 %, depending on the holding temperature during sintering, corresponding to a mass gain of 3.8 %.
The maximum allowable content of Al2O3 in sintered briquettes, which avoids the destruction of the sintered material during air storage (in the case of using hydrated lime as a source of CaO), ranges from 9.5 to 31.7 %, depending on the holding temperature during sintering, corresponding to a mass gain of 0.3 %.
Given the existing fuel units, fluxes can only be produced when hydrated lime (series 2) is utilized as a source of CaO. This is because when a mixture of hydrated lime combined with calcium carbonate (series 1) is used as a source of CaO, a holding temperature exceeding 1500 °C is required, which is unattainable with the existing fuel units, or by extending the holding time to over 25 min.
The most favorable source of CaO for flux production via the sintering method is the hydrated dust from limestone roasting furnaces. This is attributed to the fact that during sintering, its heat absorption is three times lower than that of limestone.
References
1. Sheshukov O.Yu., Mikheenkov M.A., Nekrasov I.V., Metelkin A.A., Egiazar’yan D.K. Optimization of the slag composition in ladle treatment of steel for increasing the resistance of refractories and promoting slag reuse. Metallurgist. 2018;62:723–728. https://doi.org/10.1007/s11015-018-0714-y
2. Larionov L.M., Kondratyev V.V., Kuzmin M.P. Ways of using carbon-containing waste from aluminum production. iPolytech Journal. 2017;21(4(123)):139–146. (In Russ.). https://doi.org/10.21285/1814-3520-2017-4-139-146
3. Sarkar S., Anand V., Ranjan R., Borra C.R., Sahoo P.P. Elimination of fluorspar use and reduction in lime consumption at ladle furnace by reutilizing alumina-rich ladle furnace slag. Journal of Sustainable Metallurgy. 2022;8(1):398–408. https://doi.org/10.1007/s40831-022-00492-1
4. Sheshukov O.Yu., Nekrasov I.V., Miheenkov M.A., Egiazar’yan D.K., Lobanov D.A., Neugodnikov O.V., Ivanov B.А. Experience in the use of aluminous aluminumcontaining flux during ladle processing of “aluminum-free” steels. Novye ogneupory (New Refractories). 2017;(3):75–77. (In Russ.). https://doi.org/10.17073/1683-4518-2017-3-75-77
5. Viklund-White C., Johansson H., Ponkala R. Utilization of spent refractories as slag formers in steelmaking. In: Proceedings of the 6th Int. Conf. on Molten Slags, Fluxes and Salts. Stockholm, Sweden; 2000:12–17.
6. Ramaswamy P., Gomes S.A., Ravichander N.P. Utilization of aluminum dross: Refractories from industrial waste. IOP Conference Series: Materials Science and Engineering. 2019;577(1):012101. https://doi.org/10.1088/1757-899X/577/1/012101
7. Paramguru R.K., Rath P.C., Misra V.N. Trends in red mud utilization–a review. Mineral Processing & Extractive Metallurgy Review. 2004;26(1):1–29. https://doi.org/10.1080/08827500490477603
8. Davydov S.Ya., Apakashev R.A., Koryukov V.N. The collection of nanoscale particles in alumina production. Novye ogneupory (New Refractories). 2016;(2):12–15. (In Russ.).
9. Davydov S.Ya., Apakashev R.A., Koryukov V.N. The recycling of the alumina calciner kiln dust containing nanoparticles. Novye ogneupory (New Refractories). 2014;(8):10–13. (In Russ.).
10. Nesterov A.N., Datukashvili D.O. Production of high-calcium lime in Russia. Stroitel’nye materialy. 2017;(3):52–59. (In Russ.).
11. Mantula V.D., Shaparenko A.V., Pavlyuchenko A.M., Fadeev A.V., Lyzhnik G.V. Application of bag filters in gas cleaning of limekiln and dolomite processes. Ehkologiya i promyshlennost’. 2017;(1(50)):29–35. (In Russ.).
12. Aksenova V.V., Alimbaev S.A., Pavlov A.V., Mustafin R.M. Briquetting of porous alumina-containing materials with organic binders. Izvestiya. Ferrous Metallurgy. 2021;64(5):323–329. (In Russ.). https://doi.org/10.17073/03680797-2021-5-323-329
13. Zhang Y.B., Chen X.J., Su Z.J., Liu S., Chen F., Wu N.Y., Jiang T. Improving properties of fluxed iron ore pellets with high-silica by regulating liquid phase. Journal of Iron and Steel Research International. 2021;29:1381–1392. https://doi.org/10.1007/s42243-021-00665-4
14. Fan X.H., Gan M., Jiang T., Yuan L.S., Chen X.L. Influence of flux additives on iron ore oxidized pellets. Journal of Central South University of Technology. 2010;17(4):732–737. https://doi.org/10.1007/s11771-010-0548-7
15. Li G., Jiang T., Zhang Y., Tang Z. Recrystallization of Fe2O3 during the induration of iron ore oxidation pellets. Recrystallization. 2012;13:329–350. https://doi.org/10.5772/32738
16. Umadevi T., Lobo N.F., Desai S., Mahapatra P.C., Sah R., Prabhu M. Optimization of firing temperature for hematite pellets. ISIJ International. 2013;53(9):1673–1682. https://doi.org/10.2355/isijinternational.53.1673
17. Forsmo S.P.E., Forsmo S.E., Samskog P.O., Björkman B.M.T. Mechanisms in oxidation and sintering of magnetite iron ore green pellets. Powder Technology. 2008;183(2):247–259. https://doi.org/10.1016/j.powtec.2007.07.032
18. Timofeeva A.S., Kozhukhov A.A., Nikitchenko T.V. Study of iron ore pellets hardening mechanisms in the process of burning. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 2020;76(11):1107–1112. (In Russ.). https://doi.org/10.32339/0135-5910-2020-11-1107-1112
19. Akberdin A.A., Kim A.S., Zinyakova O.N. Improvement of production technology of chromite pellets. Izvestiya. Ferrous Metallurgy. 2014;57(9):24–28. (In Russ.). https://doi.org/10.17073/0368-0797-2014-9-24-28
20. Kim A.S., Akberdin A., Isagulov A., Sultangaziev R. Relationship between phase formation processes and the quality of chromite pellets during hardening firing. Proceedings of the University. 2019;(4):24–27. (In Russ.).
21. Akberdin A.A., Kim A.S., Akberdin R.A. Agglomeration of refractory chromite ore. Proceedings of INFACON XIII - 13th Int. Ferroalloys Congress: Efficient Technologies in Ferroalloy Industry. 2020:1–4.
22.
About the Authors
V. V. AksenovaRussian Federation
Viktoriya V. Aksenova, Postgraduate of the Chair of Metallurgy of Steel, New Production Technologies and Metal Protection
4 Leninskii Ave., Moscow 119049, Russian Federation
A. V. Pavlov
Russian Federation
Aleksandr V. Pavlov, Dr. Sci. (Eng.), Prof. of the Chair of Metallurgy of Steel, New Production Technologies and Metal Protection
4 Leninskii Ave., Moscow 119049, Russian Federation
G. M. Markov
Russian Federation
Georgii M. Markov, Junior Researcher
4 Leninskii Ave., Moscow 119049, Russian Federation
Review
For citations:
Aksenova V.V., Pavlov A.V., Markov G.M. Production of refining alumina-containing fluxes by sintering from technogenic raw materials. Izvestiya. Ferrous Metallurgy. 2023;66(6):760-767. https://doi.org/10.17073/0368-0797-2023-6-760-767