Scroll to:
Evaluating the efficiency of metallized siderite concentrate electric melting
https://doi.org/10.17073/0368-0797-2023-6-653-658
Abstract
The Bakal siderites belong to low-grade refractory carbonate iron ores. The low content of phosphorus and non-ferrous metals makes siderites a valuable raw material for obtaining highly metallized concentrate suitable for use in steelmaking processes. Reduction of siderites in a rotary furnace at 1300 – 1350 °C followed by magnetic separation of waste rock allows to obtain a concentrate with metallization degree over 90 % and a content of waste rock of about 5 % suitable for steelmaking as raw materials. The purpose of this work is to evaluate the efficiency of the process aimed at obtaining metal from siderite ore including obtaining of highly metallized siderite concentrate in a recovery furnace, as well as its hot loading into ore-thermal furnace and melting process itself. To do this, the electric melting was calculated in the electric ore melting furnace providing for determination of a large number of parameters including the electricity consumption required for melting. As raw materials we used a highly metallized siderite concentrate (φmet = 92.3 %) containing 35 % of waste rock and, for comparison, a briquetted metallized siderite concentrate obtained from a lump concentrate in which a significant amount of waste rock was removed by wet magnetic separation. The results analysis shows that increase in concentrate temperatures from 25 to 1000 °C decreases specific energy consumption and at the same time increases the furnace productivity to values comparable to the parameters of melting briquetted concentrate. This confirms the efficiency of the developed process. To reduce the melting point of high-magnesium slag, it is proposed to use colemanite as flux.
Keywords
For citations:
Vusikhis A.S., Leont’ev L.I., Chesnokov Yu.A. Evaluating the efficiency of metallized siderite concentrate electric melting. Izvestiya. Ferrous Metallurgy. 2023;66(6):653-658. https://doi.org/10.17073/0368-0797-2023-6-653-658
Introduction
The Bakal group of deposits, situated in the Chelyabinsk region, holds siderites categorized as low-grade refractory carbonate iron ores. Predominantly composed of sideroplesite and pistomesite, these ores comprise iron, magnesium, and manganese carbonate solid solutions. Complementing these minerals are barren components such as quartz-clay slates, dolomites, dolomitized limestones, diabases, and quartzites [1 – 4].
Siderites, characterized by their low phosphorus content, absence of non-ferrous metals like copper and zinc, and inclusion of manganese, stand as valuable resources for yielding high-metal concentrates suitable for steelmaking processes [5; 6]. Various process solutions have been devised for their production.
Pyrometallurgical techniques for processing low-grade iron ores, involving metallization with a solid reducing agent within rotary furnaces followed by waste rock separation through grinding and magnetic procedures, are widely employed in global industrial practice [7 – 11]. The refractory nature of siderite waste rock permits operation at temperatures ranging from 1300 to 1350 °C. This facilitates the enlargement of iron grains and significantly enhances their extraction into the concentrate through magnetic separation. In this process, a product boasting a metallization degree exceeding 90 % is achieved, containing approximately 5 % waste rock primarily composed of magnesium oxide [12; 13]. The preliminary removal of easily fusible slates enables processing in heavy media [14], where during separation, the lighter fraction of waste rock floats atop the suspension and is subsequently removed.
The metallurgical plants in the Ural region are currently facing a severe shortage of iron ore resources, necessitating their importation from other regions of the country [15 – 17]. Consequently, ensuring steelmaking production with a high-quality metal charge becomes a crucial objective. Therefore, there is an urgent need to evaluate the potential utilization of metallized concentrate derived from Bakal siderites through direct reduction as a resource for electric steelmaking furnaces.
In the majority of coke-free metallurgy technologies, the final product designated for electric steelmaking typically consists of 90 – 93 % iron with a metallization degree ranging from 92 – 95 %, alongside 3 – 5 % waste rock [18; 19]. As the proportion of waste rock increases, furnace yield and productivity decline, while power consumption rises. However, it is recognized that elevating the temperature of the loaded resources leads to a significant reduction in power consumption of the arc furnace, electrodes, and refractory materials, while simultaneously enhancing furnace productivity [19].
Hence, the objective of this study is to assess the efficiency of utilizing metallized siderite concentrate within an ore-thermal furnace.
Materials and methods
The methodology for calculation and the software module developed for determining the technical and economic indicators of smelting in an ore-thermal furnace comprise a data input block, encompassing:
– chemical composition of charge materials;
– fluxing and fuel additives;
– furnace operation settings.
The electric smelting calculation in an ore-thermal furnace entails:
– determining metal and slag yield;
– assessing the chemical composition of the final slag with specified basicity or iron monoxide content;
– estimating the final metal temperature;
– designing sulfur content in the metal;
– analyzing the composition of flue gas;
– calculating mass and heat balances of the process;
– evaluating process production costs.
The developed software module, accessible in interactive mode, enables the user to:
– enter and edit information, as well as input data for calculations, with the ability to select and sort based on displayed edit fields;
– quickly add new input data by copying and editing existing records;
– archive and print both input data and calculation results, while also providing authorized access to the software module.
To determine the chemical composition of the initial materials used in the calculations, experimental modeling of the siderite metallization process in a rotary furnace was conducted. A graphite crucible containing raw siderite lumps ranging from 10 to 40 mm in size, along with breeze coke filler sized between 0 and 5 mm, was placed in a Tamman furnace heated to a temperature of 1300 °C. The setup was maintained under specified conditions for 2 h before being cooled down with the furnace. This process resulted in the formation of a metallized concentrate (in lump form). Subsequently, a portion of the metallized concentrate lumps underwent grinding and wet magnetic separation to produce a product suitable for smelting in an electric furnace in the form of briquettes, referred to as metallized concentrate (briquette). The compositions of these materials are outlined in Table 1.
Table 1. Chemical composition of metallized siderites, wt. %
|
The temperature at which the concentrate (metallized concentrate in lump form) was loaded into the furnace after reducing roasting varied within the range of 25 to 1000 °С. The concentrate (metalized concentrate in briquette from) was loaded at 25 °С.
Results and discussion
Variants of calculations of melting indicators in an ore-thermal furnace with the use of lump metallized siderite concentrate loaded into the furnace at temperatures of 25 – 1000 °С as an initial resource, all other conditions being equal, are given in Table 2.
Table 2. Indicators of melting in ore-thermal furnace
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
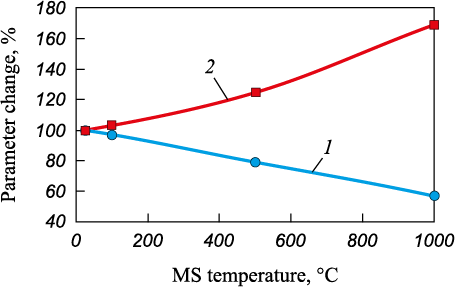
Graphical interpretation of the effect of temperature of the lump metallized siderite concentrate loaded into a furnace on the productivity and specific power consumption for melting is given in the Figure.
Influence of initial temperature of metallized siderite (MS) |
The analysis of the obtained results shows that the use of highly heated lump metallized siderite concentrate is one of the important process measures to increase the efficiency of its electric smelting. When the temperature of the material increases in the range from 25 to 1000 °С, the specific power consumption decreases and the furnace productivity increases.
Table 3 presents calculations comparing the melting indicators in an ore-thermal furnace using a briquetted metallized siderite concentrate loaded at a temperature of 25 °C as feedstock, with all other conditions held constant. Additionally, the data obtained, along with the calculated melting indicators of the lump metallized siderite concentrate loaded at 1000 °C, are provided.
Table 3. Comparative indicators of melting in ore-thermal furnace
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
An analysis of the data reveals that feeding a lump metallized siderite concentrate directly into an ore-thermal furnace immediately after discharge from the rotary furnace at temperatures exceeding 1000 °C (similar to the technology employed in processing titanomagnetites of the Bushveld complex in roasting furnaces with loading of a hot stub end into the ore-thermal furnace [20]), proves to be more efficient compared to melting a briquetted metallized siderite concentrate produced through the pyrometallurgical processing of siderites as described in [6; 12; 13].
Consequently, the pyrometallurgical processing technology eliminates the need for operations such as grinding, magnetic separation for waste rock removal, as well as drying and briquetting, thereby significantly reducing the product’s overall cost.
The calculations demonstrate that the melting process yields slag with a high magnesium oxide content, characterized by a high melting temperature. However, the addition of material containing boron, such as colemanite [21], to high-magnesia steelmaking slags significantly reduces their melting point.
Conclusions
The direct loading of lump metallized siderite concentrate into the electric furnace from the reducing furnace in a hot state (at temperatures exceeding 1000 °C) proves to be an effective method for melting and producing metal – a semi-product suitable for subsequent steel production. To ensure adequate fluidity of the high-magnesia slag at outlet temperatures, the addition of boron-containing materials, such as colemanite, to the charge becomes necessary.
References
1. Akhlyustina A.I. Crystal-chemical changes in sideroplesite during firing of Bakal iron ore. Gornyi zhurnal. 1968;(8): 60–61. (In Russ.).
2. Khokhlov D.G., Akhlyustina A.I. Technology of Bakal carbonate iron ores preparation for blast furnace smelting. Stal’. 1968;(4):289–294. (In Russ.).
3. Akhlyustina A.I., Zhukovskii G.V., Kvaskov A.P. Technological classification of iron ores of the Bakal deposit. Trudy “Uralmekhanobr”. 1972;18:37–43. (In Russ.).
4. Krasnoborov V.A., Yaroshevskii S.L., Denisov A.A., etc. Efficiency and Prospects of Application of Siderite Ore in Blast Furnace Smelting. Donetsk; 1996:87. (In Russ.).
5. Yur’ev B.P., Melamud S.G., Spirin N.A., Shatsillo V.V. Technological and Thermal Engineering Bases of Siderite Ore Preparation for Metallurgical Processing: Monograph. Yekaterinburg: Den’ RA; 2016:428. (In Russ.).
6. Vusikhis A.S., Leont’ev L.I. Application of Siderite Ores in Iron and Steel Production: Monograph. Moscow; Vologda: Infra-Inzheneriya; 2022:116. (In Russ.).
7. Tatsienko P.A. Preparation of Refractory Iron Ores. Moscow: Nedra; 1979:208. (In Russ.).
8. Tulin N.A., Kudryavtsev V.S., Pchelkin S.A., etc. Development of Coke-Free Metallurgy. Moscow: Metallurgiya; 1987:228. (In Russ.).
9. Kurunov I.F., Savchuk N.A. State and Prospects of Direct Metallurgy of Iron. Moscow: Chermetinformatsiya; 2002:186. (In Russ.).
10. Bondarenko B.I., Shapovalov V.A., Garmash N.I. Theory and Technology of Coke-Free Metallurgy. Kiev: Naukova Dumka; 2003:536. (In Russ.).
11. Shumakov N.S. Production of metallized concentrate from siderite ores of the Bakal deposit. Kompleksnoe ispol’zovanie mineral’nogo syr’ya. 1990;(4):52–55. (In Russ.).
12. Vusikhis A.S., Dvinin V.I., Dmitriev A.N., Kashin V.V., Leont’ev L.I., Nafikov R.A., Chentsov A.V., Shavrin S.V. Evaluating the efficiency of producing highly metallized concentrate from Bakal siderites by hot reduction in a rotary kiln. Metallurgist. 2001;45(1-2):14–19. https://doi.org/10.1023/A:1010495309806
13. Vusikhis A.S., Sheshukov O.Yu., Leont’ev L.I. Method of metallization of magnesium-containing carbonate iron ore materials. Patent RF no. 2489494, MPK В21С 1/00. Bulleten’ izobretenii. 2013;(22). (In Russ.).
14. Zhunev A.G., Yur’ev B.P., Blank M.E. Intensification of firing and agglomeration of siderite ores. Ferrous Metallurgy. Bulletin of Scientific, Technical and Economic Information. 1988;(3):2–13. (In Russ.).
15. Pakhomov V.P., Dushin A.V. Analysis of the mineral-raw material safety in the Ural Federal District. Ekonomika regiona. 2008;(3(15)):129–143. (In Russ.).
16. Valiev N.G., Slavikovskii O.V., Slavikovskaya Yu.O. Features of development of mineral resource base in the Urals urbanized territories. Gornyi informatsionno-analiticheskii byulleten’ (nauchno-tekhnicheskii zhurnal). 2012;(6):344–347. (In Russ.).
17. Kornilkov S.V., Kantemirov V.D. Iron ore deposits of the Nether-Urals as a prospective raw materials base for the Urals metallurgy. Izvestiya vuzov. Gornyi zhurnal. 2015;(8);22–28. (In Russ.).
18. Soifer V.M. The use of products of direct iron reduction in electric furnaces. Novosti chernoi metallurgii za rubezhom. 2007;(8):33–35. (In Russ.).
19. Timoshpol’skii V.I., Trusova I.A., Plushchevskii I.N., Korneev S.V. Prospects for production and use of metallized raw materials for obtaining high-quality steel grades. Message 1. Analysis of modern schemes for obtaining metallized raw materials. Lit’ye i metallurgiya. 2009;(1(50)):134–138. (In Russ.).
20. Rohmann B., Raper A.G. Recovery of vanadium from hot metal using the shaking ladle process a preliminary report. Journal of Iron and Steel Research International. 1970;208(4):336–342.
21. Vusikhis A.S., Agafonov S.N., Tyushnyakov S.N., Sergeeva S.V., Leont’ev L.I. Influence of boric anhydride on viscosity and melting point of high-magnesium steelmaking slags. In: Fundamental Research and Applied Development of Processing and Disposal of Technogenic Formations: Proceedings of the VI Congress with Int. Part. TECHNOGEN 2023. Yekaterinburg: Ural Branch of the RAS; 2023: 351–353. (In Russ.). https://doi.org/10.34923/technogen-ural.2023.15.21.001
About the Authors
A. S. VusikhisRussian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. I. Leont’ev
Russian Federation
Leopol’d I. Leont’ev, Academician, Adviser, Russian Academy of Sciences, Dr. Sci. (Eng.), Prof., National University of Science and Technology “MISIS”, Chief Researcher, Institute of Metallurgy, Ural Branch of the Russian Academy of Science
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
32a Leninskii Ave., Moscow 119991, Russian Federation
Yu. A. Chesnokov
Russian Federation
Yurii A. Chesnokov, Cand. Sci. (Eng.), Chief Specialist
8 Osnovinskaya Str., Yekaterinburg 620041 Russian Federation
Review
For citations:
Vusikhis A.S., Leont’ev L.I., Chesnokov Yu.A. Evaluating the efficiency of metallized siderite concentrate electric melting. Izvestiya. Ferrous Metallurgy. 2023;66(6):653-658. https://doi.org/10.17073/0368-0797-2023-6-653-658