Scroll to:
Effect of hydrogen on nickel oxide reduction on the surface of nozzle blade of a gas turbine unit
https://doi.org/10.17073/0368-0797-2023-5-604-609
Abstract
Currently, there is a growing interest in the use of hydrogen in the composition of fuel mixtures for turbojet engines and gas turbine units (GTU). The effect of hydrogen on heat-resistant nickel alloys of gas turbine blades has been little studied. In this regard, this work is devoted to studying the effect of hydrogen on nickel oxide reduction on the surface of the nozzle blade of a gas turbine engine. Hydrogen is a good reducing agent. Therefore, this article discusses the effects of hydrogen under various conditions with metal oxides, and methods of metal oxides reduction on the surface of the blades of a gas turbine engine. The thermodynamics of the interaction of aluminum, titanium, nickel and tungsten oxides with hydrogen fluoride and reactions of fluoride with hydrogen was investigated in the temperature range 273 – 1373 K. It was established that the interaction of aluminum oxide with hydrogen fluoride occurs in the temperature range from 273 to 1073 K, titanium oxide with hydrogen fluoride – from 273 to 373 K, nickel oxide with hydrogen fluoride – from 273 to 873 K. In this case, of the resulting fluorides, only nickel fluoride interacts with hydrogen at temperatures above 673 K. Hydrogen interacts with nickel oxide throughout the entire temperature range, and with tungsten oxide at temperatures above 1173 K. We studied the effect of hydrogen on heat-resistant nickel alloys of gas turbine blades subjected to preliminary fluorination and not treated with fluorine compounds. Nickel oxide reduction with hydrogen proceeds better after the preliminary fluorination process. In this case, particles 2 – 5 μm in size containing 90.16 % Ni are formed on the surface of the blade sample. Without fluorination, this process at 1223 K and duration of 1 h does not occur.
Keywords
For citations:
Fomina D.D., Poilov V.Z., Gallyamov A.N. Effect of hydrogen on nickel oxide reduction on the surface of nozzle blade of a gas turbine unit. Izvestiya. Ferrous Metallurgy. 2023;66(5):604-609. https://doi.org/10.17073/0368-0797-2023-5-604-609
Introduction
Hydrogen finds extensive application across chemical, power generation, and metallurgical industries as a versatile fuel, effectively curbing carbon dioxide emissions into the atmosphere. It serves as an energy reservoir, a chemical reagent for synthesizing organic compounds, and a potent reducing agent, among other roles. Although hydrogen is typically chemically inert, its reactivity amplifies, especially when subjected to heat. Under these conditions, molecular hydrogen engages in chemical interactions with various metals, non-metals, and complex compounds. The heightened chemical activity of hydrogen, influenced by additional factors, stems from the partial formation of atomic hydrogen, significantly more reactive than its molecular counterpart. Notably, hydrogen proves to be an exceptional agent for reducing metal oxides into metals. Both atomic and molecular hydrogen can carry out reducing functions [1 – 3].
Atomic hydrogen interacts with the surfaces of solids upon heat absorption, necessitating consideration of different substances’ propensity to interact with hydrogen atoms. The recombination of hydrogen atoms occurs most efficiently due to the catalytic effects of metals, with activity decreasing in the following order: Pt > Pd > W > Fe > Cr > Ag > Cu > Pb.
Additionally, the recombination of hydrogen atoms (involving the capture of a free electron by an ion) occurs on the surfaces of oxides such as MgO, CaO, BaO, Al2O3 , Cr2O3 [4].
An important aspect involves exposing the surface of a metal covered with an oxide film to molecular hydrogen, thereby eliminating this film by reducing the oxides to their metallic state. This study delves into the impact of hydrogen on the surfaces of alloy components used in gas turbine unit (GTU) blades, examining both theoretical implications and practical application.
The effectiveness of hydrogen’s reducing capabilities is detailed in [5], wherein the authors explore the reduction of various metal oxides. Atomic hydrogen, generated on a heated tungsten catalyst, is employed for the reduction process. The study revealed that oxides of Cu, Ru, Nb, Mo, Rh, Pd, Ir and Pt can be reduced by atomic hydrogen at a substrate temperature of 313 K.
Studies conducted in [6; 7] investigated the extraction processes of nickel from serpentine and limonite ores through leaching at elevated temperatures and pH = 13, followed by the reduction of nickel salts using hydrogen. Exploring the use of hydrogen plasma [8], generated via inductive radio frequency glow discharge at 27.12 MHz and 700 W RF generator power, at a density of 1 – 3\(^{(10 – 15)}\) m\(^–\)3 within hydrogen pressures ranging from 0.05 to 50 Pa, holds significance for copper oxide reduction. Researchers in [9] similarly employed the reduction process in Н2 plasma for phosphide synthesis. Remarkably, high efficiency was attained in hydrogen plasma due to hydrogen’s heightened reactivity in reduction processes.
The ability to reduce iron (III) oxide in a hydrogen-rich atmosphere has been investigated, revealing that an insufficient supply of hydrogen impedes the completion of the reduction process [10]. Additionally, hydrogen plays a crucial role in restoring cathode materials within lithium-ion batteries [11].
In examining methods for cleaning nozzle blades as outlined in [12; 13], the reducing properties of hydrogen are leveraged in a stage aimed at cleansing the surface of gas turbine engine (GTE) blades from metal oxides, using elemental fluorine [14; 15]. This method is employed to clean damaged metal parts composed of a heat-resistant nickel alloy containing microcracks, facilitating part repair via soldering. Elemental fluorine acts as an intermediary reagent, eliminating metal oxides from heat-resistant nickel alloys and forming metal fluorides with the alloy components. The source of elemental fluorine originates from the products resulting from the thermal decomposition of fluorocarbon resin. Subsequently, the reduction of metal fluorides is carried out using hydrogen at elevated temperatures.
The treated part exhibits a surface layer devoid of oxides. It has been noted that the surface contains trace amounts of titanium and aluminum, enhancing the ease of soldering the part.
The patents [16; 17] outline a process involving the exposure of a part to an atmosphere containing carbon, oxygen (in the form of carbon monoxide), hydrogen, and fluorine (С – О – Н – F). Initially, these patents detail the decomposition process of polytetrafluoroethylene (PTFE), resulting in the formation of tetrafluoroethylene monomer, which subsequently reacts with hydrogen, producing hydrogen fluoride.
Distinguishing themselves from [14; 15], patents [16; 17] operate within a distinct temperature range of 973 – 1073 K, with a duration of 4 h within this gas environment. During this phase, metal fluorides are generated. In a subsequent step, hydrogen is applied to the part’s surface within a temperature range of 1223 – 1373 K.
Existing publications lack an assessment of the efficiency in reducing metal oxides of heat-resistant nickel alloys solely subjected to prior fluorination and not treated with fluorine compounds. Therefore, this study aims to elucidate the characteristics of the process involved in reducing metal oxides of heat-resistant nickel alloys with hydrogen subsequent to preliminary fluorination and in the absence of further treatment with fluorine compounds.
Thermodynamic analysis results
Thermodynamic analysis of fluorination
The thermodynamic analysis conducted involved the examination of the interaction between aluminum, titanium, nickel, and tungsten oxides with hydrogen fluoride, as well as the subsequent reaction of fluorides with hydrogen. The following thermodynamic characteristics pertain to the reducing potential of hydrogen during its interaction with the oxide film present on the surface of the heat-resistant nickel alloy GTU. Presented here are the primary reactions that may occur during these processes, along with enthalpy and entropy values obtained from the reference book [18].
| Al2O3 + 6HF(g) = 2AlF3 + 3H2O; | (1) |
| TiO2 + 4HF(g) = TiF4 + 2H2O; | (2) |
| NiO + 2HF(g) = NiF2 + H2O; | (3) |
| WO3 + 6HF(g) = WF6(g) + 3H2O. | (4) |
The examination of the Gibbs energy of reactions (1) – (4) indicates specific temperature ranges for interactions between aluminum oxide and hydrogen fluoride (273 to 1073 K), titanium oxide and hydrogen fluoride (273 to 373 K), and nickel oxide and hydrogen fluoride (273 to 873 K), as confirmed by their respective negative Gibbs energy values. Notably, the reaction between tungsten oxide and hydrogen fluoride does not occur.
It’s noted that above 873 K, aluminum fluoride sublimates from the surface of the GTE blade. A similar sublimation phenomenon occurs when titanium fluoride is heated to temperatures higher than 353 K [19].
Thermodynamic analysis of metal fluoride-hydrogen reactions
The interaction of metal fluorides with hydrogen can proceed according to the following reactions
| 2AlF3 + 3H2 = 2Al + 6HF; | (5) |
| TiF4 + 2H2 = Ti + 4HF; | (6) |
| NiF2 + H2 = Ni + 2HF. | (7) |
Analysis of the Gibbs energy indicates that reactions (5) and (6) do not occur as their corresponding Gibbs energy values are positive. Additionally, the reduction of nickel fluoride (7) takes place at temperatures equal to or higher than 673 K.
Thermodynamic analysis of metal oxides-hydrogen reactions
The interaction of metal oxides with hydrogen can proceed according to the following reactions
| Al2O3 + 3H2(g) = 2Al + 3H2O(g) ; | (8) |
| TiO2 + 2H2(g) = Ti + 2H2O(g) ; | (9) |
| NiO + H2(g) = Ni + H2O(g) ; | (10) |
| WO3 + 3H2(g) = W + 3H2O(g) . | (11) |
Analyzing the Gibbs energy of reactions (8) – (11) reveals that hydrogen reduces nickel oxide across the entire temperature range under consideration. Additionally, tungsten oxide reacts with hydrogen at temperatures exceeding 1173 K. This aligns with existing literature data1, which specifies that the reduction process of tungsten anhydride takes place in a hydrogen flow with moisture content not exceeding 2 g/m3 and oxygen content not surpassing 0.4 vol. %.
Experimental
The study utilized a gas turbine engine nozzle blade provided by Perm Motors JSC as the subject of investigation.
Potassium bifluoride served as the source to generate hydrogen fluoride and facilitate the surface fluorination process. Thermal decomposition of potassium bifluoride occurred at temperatures exceeding 1023 K within a single-zone tube furnace manufactured by Protherm furnaces company. The fluorination process was conducted at a temperature of 1023 K for a duration of 2 h within an oxygen-free environment. To achieve this, the reactor underwent purging with argon, which was purified to eliminate traces of oxygen using copper chips and titanium sponge at temperatures up to 1073 K. After fluorination, the sample surface underwent reduction by hydrogen supplied from a hydrogen generator. This reduction process occurred at a temperature of 1223 K for a duration of 1 h. Subsequently, the sample was cooled to room temperature in an oxygen-free environment. The surface analysis of the sample was conducted using an S-3400N electron scanning microscope manufactured by HITACHI, Japan, equipped with an attachment from Bruker (Germany) for X-ray spectral and X-ray fluorescence analyses.
Results and discussion
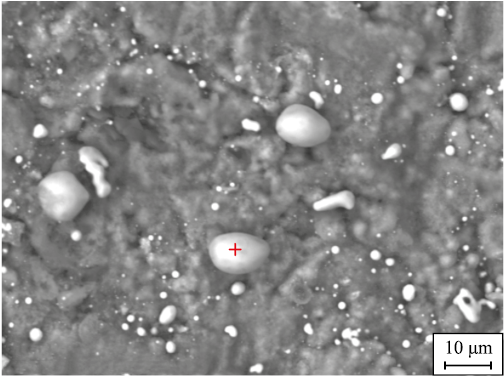
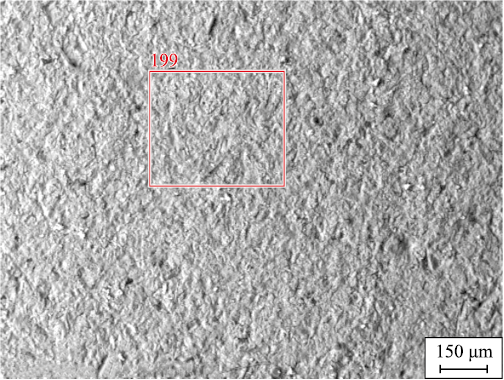
The description provides an analysis of the blade surface post-fluorination and hydrogen reduction, illustrated in Fig. 1, highlighting the presence of small white-colored inclusions indicative of nickel particles. At a higher magnification (Fig. 2), the spherical nature of these nickel particles becomes apparent.
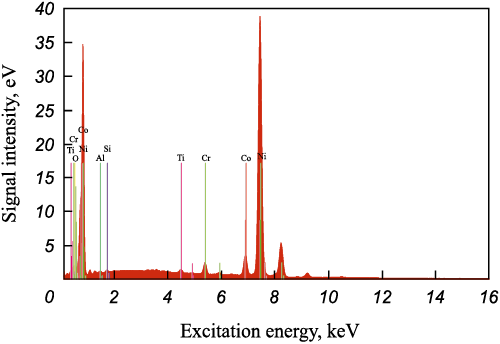
The composition analysis depicted in Fig. 3 confirms that these spherical particles, ranging in size from 2 to 5 μm, contain 90.16 % Ni. This observation leads to the conclusion that hydrogen effectively reduces nickel fluoride to elemental metallic nickel.
Fig. 1. Micrograph of the blade sample surface
Fig. 2. Micrograph of nickel particles on the blade sample surface
Fig. 3. Spectrum of a nickel particle on the blade sample surface |
In contrast, an experiment examining the interaction of a non-fluorinated sample with hydrogen at a temperature of 1223 K for a duration of 1 h showed different results, as depicted in Fig. 4. Unlike the previous case (Fig. 1), no nickel particles are observed on the surface of the blade. This absence of nickel reduction by hydrogen is contrary to the thermodynamic possibility of nickel oxide reduction with hydrogen. It was noted in [20] that structural and functional materials based on chromium and nickel demonstrate considerable resistance to hydrogen at both normal and elevated temperatures. The hindrance to the nickel reduction process with hydrogen seems to stem from kinetic limitations rather than thermodynamic factors. This limitation likely arises due to a dense film of aluminum, titanium, and tungsten oxides present on the sample surface. This film appears to impede the process of hydrogen reduction of nickel oxide. When hydrogen fluoride interacts with the dense film comprising aluminum, titanium, and tungsten oxides, it initiates the destruction of this film. This promotes the reduction process by providing hydrogen the necessary access to the nickel oxide reaction zone.
Fig. 4. Micrograph of the blade sample surface after hydrogen exposure |
Conclusions
A comprehensive thermodynamic analysis investigated the interaction of aluminum, titanium, nickel, and tungsten oxides with hydrogen fluoride across temperatures ranging from 273 to 1373 K. The findings revealed distinct temperature ranges for each oxide’s interaction with hydrogen fluoride: aluminum oxide from 273 to 1073 K, titanium oxide from 273 to 373 K, and nickel oxide from 273 to 873 K. Notably, among the resulting fluorides, only nickel fluoride displayed reactivity with hydrogen above 673 K. Hydrogen reacts with nickel oxide over the entire temperature range, and with tungsten oxide at temperatures above 1173 K.
Further experimental observation showed that the reduction of nickel oxide occurred through fluorination followed by hydrogen reduction at 1223 K over a 1 h duration. This process resulted in the formation of 2 – 5 μm particles containing 90.16 % Ni on blade surfaces. Importantly, without fluorination, the reduction process of nickel oxide did not occur. This discrepancy was attributed to the presence of a dense film composed of aluminum, titanium, and tungsten oxides on the sample surface, impeding the hydrogen reduction of nickel oxide. Exposure to hydrogen fluoride disrupted this film by generating volatile aluminum and titanium fluorides, allowing hydrogen access to the nickel oxide reduction zone.
References
1. Radchenko R.V. Hydrogen in Energy Sector. Yekaterinburg: Izd-vo ural. un-ta; 2014:229. (In Russ.).
2. Okonskii I.S., Osokin A.A., Fedyukov Yu.S. Processes and Devices of Oxygen and Cryogenic Production. Moscow: Mashinostroenie; 1985:256. (In Russ.).
3. Ugai Ya.A. General and Inorganic Chemistry. Moscow: Vyssh. shk.; 1997:527. (In Russ.).
4. Ioffe V.B. Basics of Hydrogen Production. Leningrad: gos. nauchno-tekhnicheskoe izdatel’stvo neftyanoi i gorno-toplivnoi literatury; 1960:430. (In Russ.).
5. Izumi A., Ueno T., Miyazaki Y., Oizumi H., Nishiyama I. Reduction of oxide layer on various metal surfaces by atomic hydrogen treatment. Thin Solid Films. 2008;516(2-4):853–855. https://doi.org/10.1016/j.tsf.2007.06.097
6. Bryson J.P., Distin P.A. The recovery of nickel from laterrites by chelate formation and reduction with hydrogen. Hydrometallurgy. 1978;3(4):343–354. https://doi.org/10.1016/0304-386X(78)90038-5
7. Crundwell F.K., Moats M.S., Ramachandran V., Robinson T.G., Davenport W.G. Chapter 27 – Hydrogen reduction of nickel from ammoniacal sulfate solution. Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals. 2011:347–354. https://doi.org/10.1016/B978-0-08-096809-4.10027-9
8. Brecelj F., Mozetic M. Reduction of metal oxide thin layers by hydrogen plasma. Vacuum. 1990;40(1-2):177–181. https://doi.org/10.1016/0042-207X(90)90149-S
9. Guan J., Wang Y., Qin M., Yang Y., Li X., Wang A. Synthesis of transition-metal phosphides from oxidic precursors by reduction in hydrogen plasma. Journal of Solid State Chemistry. 2009;182(6):1550–1555. https://doi.org/10.1016/j.jssc.2009.03.026
10. Jabbour K., El Hassan N. Optimized conditions for reduction of iron (III) oxide into metallic form under hydrogen atmosphere: A thermodynamic approach. Chemical Engineering Science. 2022;252:117297. https://doi.org/10.1016/j.ces.2021.117297
11. Shanker Bhandari G., Dhawan N. Gaseous reduction of NMC-type cathode materials using hydrogen for metal recovery. Process Safety and Environmental Protection. 2023;172: 523–534. https://doi.org/10.1016/j.psep.2023.02.053
12. Loginova D.I., Fomina D.D., Fedotova O.A., Poilov V.Z. Methods of cleaning the nozzle blades of a gas turbine engine from metal oxides. Vestnik PNIPU. Khimicheskaya tekhnologiya i biotekhnologiya. 2023;(1):19–34. (In Russ.). https://doi.org/10.15593/2224-9400/2023.1.02
13. Fomina D.D., Poilov V.Z. Methods of cleaning the surface of blades of gas turbine engines from carbon deposits and oxidation products. In: All-Russ. Sci. and Pract. Conf. with Int. Part. “Chemistry. Ecology. Urban Studies” April 28–29, 2022, Perm. Perm: PNIPU; 2022:191–194.
14. Cyhasteen J.W. Method for cleaning metal parts with elemental fluorine. Patent US no. 4188237. CPC B23K 1/206. Publ. 12.02.1980.
15. Cyhasteen J.W. Method for cleaning metal parts. Patent US no. 4324594. CPC C23G 5/00. Publ. 13.04.1982.
16. Cyhasteen J.W. Process for removing protective coatings and bonding layers from metal parts. Patent US no. 5071486. CPC C23F 1/44. Publ. 10.12.19911.
17. Pai Z.P., Parmon V.N., Pai V.V., Fedotenko M.A., Iakovlev I.V., Shangina A.B. Contact solution, method and installation for cleaning the surface of metal alloys, including the surface of cracks and narrow gaps. Patent RF no. 2419684. Bulleten’ izobretenii. 2013;(33). (In Russ.).
18. Ravdelya A.A., Ponomareva A.M. Brief Reference Book of Physico-Chemical Quantities. St. Petersburg: “Ivan Fedorov”; 2003:240. (In Russ.).
19. Myasoedov B.F. Methods of Substances Concentration in Analytical Chemistry. Moscow: Nauka; 1965:394. (In Russ.).
20. Fomina D.D., Poilov V.Z. Study of new structural and functional materials and coating resistant to hydrogen environment. Vestnik PNIPU. Khimicheskaya tekhnologiya i biotekhnologiya. 2022;(2):55–72. (In Russ.). https://doi.org/10.15593/2224-9400/2022.2.04
About the Authors
D. D. FominaRussian Federation
Dar’ya D. Fomina, Assistant of the Chair of ”Chemical Engineering”
29 Komsomolskii Ave., Perm 614990, Russian Federation
V. Z. Poilov
Russian Federation
Vladimir Z. Poilov, Dr. Sci. (Eng.), Prof. of the Chair “Chemical Engineering”, Head of the Shared Collective Use Center “Center for High-tech Chemical Technologies and Physico-Chemical Research”
29 Komsomolskii Ave., Perm 614990, Russian Federation
A. N. Gallyamov
Russian Federation
Andrei N. Gallyamov, Postgraduate of the Chair “Chemical Engineering”
29 Komsomolskii Ave., Perm 614990, Russian Federation
Review
For citations:
Fomina D.D., Poilov V.Z., Gallyamov A.N. Effect of hydrogen on nickel oxide reduction on the surface of nozzle blade of a gas turbine unit. Izvestiya. Ferrous Metallurgy. 2023;66(5):604-609. https://doi.org/10.17073/0368-0797-2023-5-604-609
JATS XML