Scroll to:
Effect of silver and heat treatment on properties of 03Kh17N10M2 austenitic steel wire
https://doi.org/10.17073/0368-0797-2023-5-544-553
Abstract
The article examines the influence of various heat treatments, their temperature, as well as silver alloying on mechanical properties, phase composition and structure of steel wire from chromium-nickel-molybdenum austenitic stainless steel 03Kh17N10M2. Choice of the amount of silver alloying was based on previous studies of the antibacterial effect of modifying medical steels with silver. Since the antibacterial effect was confirmed on several bacterial strains, for the most efficient operation of alloys, it is necessary to determine the best temperature mode for working with them. Steel for the study was smelted and then transformed into wire through rolling, forging and drawing operations. On the obtained wire samples of different diameters with a silver content (0; 0.2 and 0.5 wt. %) mechanical tests were carried out to determine the elongation, yield strength and tensile strength. Various modes and temperatures of heat treatment were tested on wire of different diameters to study their effect on mechanical properties and structure. Microstructure of the wire samples subjected to heat treatment and obtained after drawing was investigated. A phase analysis was also carried out to determine the effect of silver in various quantities on austenitic steel. According to the results of the phase composition analysis, it was concluded that silver reduces the amount of gamma phase in steel, and this effect increases in proportion to the increase in silver amount. This change correlates with a slight drop in the metal ductility. At the same time, there are no significant changes in the strength characteristics and microstructure from the presence of silver.
For citations:
Gorbenko A.D., Kaplan M.A., Konushkin S.V., Nasakina E.O., Baikin A.S., Sergienko K.V., Ivannikov A.Yu., Morozova Ya.A., Oshkukov S.A., Kolmakov A.G., Sevost’yanov M.A. Effect of silver and heat treatment on properties of 03Kh17N10M2 austenitic steel wire. Izvestiya. Ferrous Metallurgy. 2023;66(5):544-553. https://doi.org/10.17073/0368-0797-2023-5-544-553
Introduction
Austenitic steels find widespread use in economic sectors where materials necessitate high resistance to corrosion and durability. These sectors include medicine, the food industry, chemical production, among others. This utilization is linked to a specific set of requirements, primarily corrosion resistance and relatively low cost. These steels have gained significant traction in medicine, particularly in applications involving direct and prolonged contact with the human body, such as implantation. This is attributed to their biotolerance and relatively high plasticity [1 – 3].
For short-term implantation, biotolerant materials are employed, meeting the standards set by State Standards GOST, such as high-alloy stainless steels [4; 5]. While they can be utilized in creating long-lasting prostheses [6; 7], current practices involve augmenting these materials with coatings and other methods to enhance biocompatibility [8]. Stainless medical steels exhibit resistance to the aggressive internal environment of the human body and, notably, do not typically induce an immune reaction, barring individual, rare allergic responses to specific components. However, despite the advantages of these materials, the possibility of bacterial infection in the vicinity of the implant cannot be entirely ruled out during operations [9 – 12].
Silver is known for its capability to disrupt bacteria metabolism [13 – 16]. This essential property persists when silver is incorporated into coatings [17; 18] or utilized as a doping component [19 – 21]. Several publications [2; 19] detail the authors’ endeavors in producing 03Kh17N10M2 steel with 0.2 and 0.5 % Ag additions, examining these compositions for their antibacterial properties. The research revealed that a mere 0.2 % Ag within the steel composition suffices to suppress detrimental strains of Pseudomonas marginalis and Clavibacter bacteria. Furthermore, an escalation in silver content resulted in a more pronounced effect. Additionally, these compositions underwent scrutiny to ascertain their mechanical properties and microstructural changes. However, the research focused on materials in the form of ingots and rolled products, while acknowledging the potential use of such steels in wire form or as a workpiece. This could facilitate further utilization in additive manufacturing, welding, or product formation through simple mechanical processing.
This study aimed to determine the mechanical properties of wire fabricated from 03Kh17N10M2 steel (akin in chemical composition to steels employed in medicine and jewelry, such as 316L) with silver additives. The investigation explored the influence of silver on the steel’s structure, phase composition, mechanical properties, and the impact of various heat treatment methods on the silver-enhanced steel.
Materials and methods
The smelting of steel took place at the Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences. Through a process of triple remelting, chromium-nickel-molybdenum stainless austenitic steel 03Kh17N10M2 was produced, incorporating additional doping with silver. The chemical composition of the resulting alloys is detailed in Table 1. Alloy 1 represents the base composition without the addition of silver, while alloy contains 0.2 % Ag and alloy 3 contains 0.5 % Ag. Further details on the ingot production technology are available in [2].
Table 1. Compositions of the smelted alloys
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
The cast billets were rolled into 1 mm thick plates using a two-roll mill. Subsequently, the deformed workpieces were rotated by 90° and, through repeated rolling, were shaped into bars measuring 10×10 mm. To achieve a diameter of 2.4 mm, rotational forging was conducted on radial forging machines. This process involved successive changes of strikers, progressing in increments of 1 mm until reaching a 5 mm diameter, at which point the increment was reduced to 0.5 mm. Intermediate heating up to 700 °C was applied during the forging process.
In preparation for subsequent operations and to analyze the impact of various heat treatments (HT) on the properties of the resulting steel bars, a bar with a 2.4 mm diameter underwent different heat treatment processes–annealing, normalization, and quenching – inside a muffle furnace.
Before reducing the diameter further, a scale removal process was performed using a solution of nitric and hydrochloric acids. Subsequently, the bars were lubricated with sodium soap, and a layer of borax was applied as a lubricating agent to enhance adhesion to the steel surface.
The subsequent reduction in diameter to 1 mm was achieved utilizing a drawing machine in an atmospheric environment. The wire underwent processing at a speed of 5 m/min, gradually decreasing in diameter by 0.2 mm per pass, from 2.4 to 1.6 mm. Following this, a two-minute heat treatment at 900 °C was conducted in the furnace to anneal the cold-worked steel. Subsequent wire drawing to reduce the diameter to 1 mm occurred at half the previous steps and speeds: 0.1 mm per pass at a rate of 2.5 m/min.
Upon achieving the final diameter, the silver-free steel wire underwent heat treatment at temperatures of 900, 950, 1000 and 1050 °C, each for a holding time of 2.5 min (Fig. 1).
Fig. 1. Obtained wire with a diameter of 1 mm |
Structural examinations were performed on thin sections of the resulting steel samples. These samples were embedded in non-conductive resin, followed by grinding and polishing.
Surface etching was conducted using a composition suitable for high-alloy steels, comprising hydrofluoric, sulfuric, and nitric acids (2, 15 and 5 %, respectively, with the rest being water).
Microstructural analyses were carried out using an Altami MET 5C microscope, resulting in images depicting the wire’s structure at two different diameters: 2.4 and 1 mm. Photographic recording was executed in polarized light with maximum brightness.
The phase composition of the resulting steels was investigated through X-ray diffraction patterns obtained using CuKα radiation in a parallel beam geometry. The positional error of reflections during analysis did not exceed 0.01° 2θ. The crystal lattice parameter was adjusted by extrapolation to θ = 90° using the Nelson–Riley method within Origin-2017 software. Microstrain in the crystal lattice of the main phase was determined using the Williamson–Hall method, and the quantitative content of crystalline phases was estimated using the corundum number method.
Mechanical properties of the resulting wires were calculated based on tensile tests conducted on an INSTRON 3382 universal testing machine. The average values were derived from five experiments, determining ultimate strength, yield stress, and relative elongation in accordance with State Standard GOST 1497 – 84, utilizing the software integrated with the testing machine.
Results and discussion
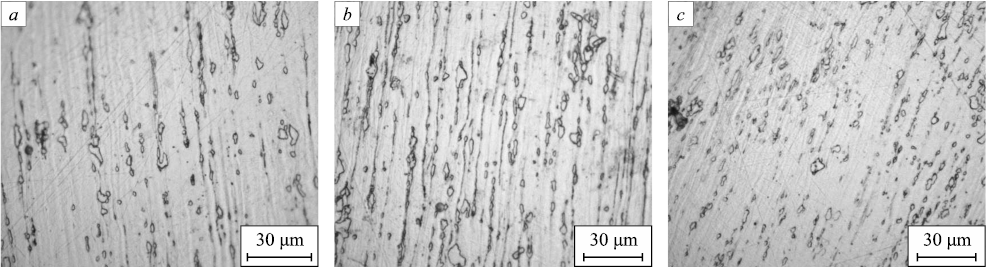
Fig. 2 displays the surface characteristics of sections obtained from bars with a 2.4 mm diameter.
Fig. 2. Microstructure of the bars: |
Upon analyzing the microstructure, it can be inferred that the presence of silver did not exhibit a discernible impact on the grain size in both cases.
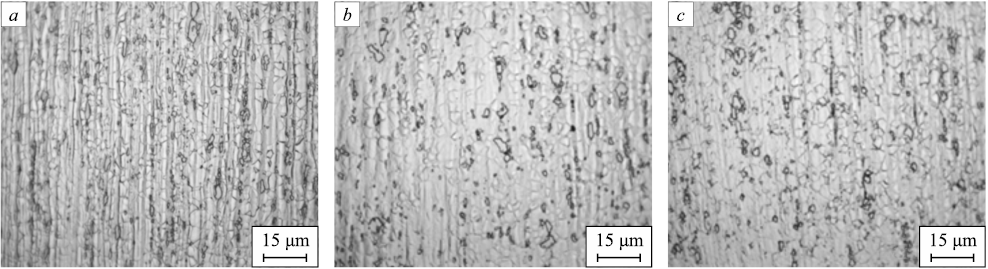
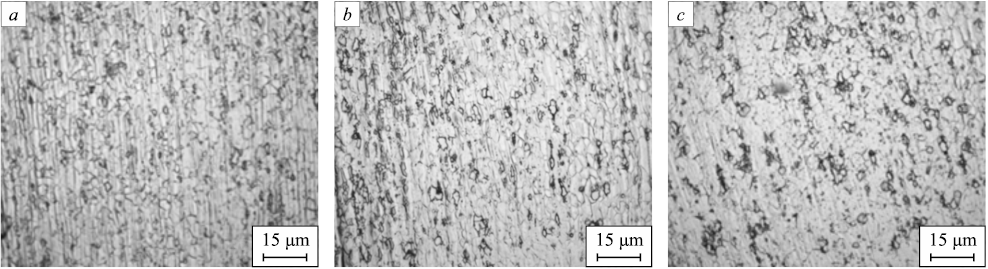
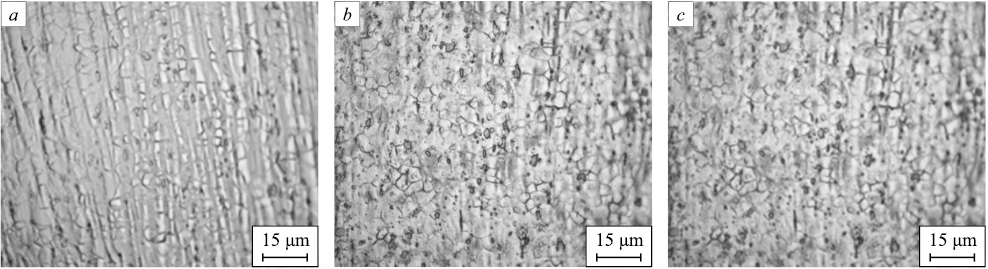
The materials after drawing are strengthened, heavily deformed throughout the volume of the metal, thereby exhibiting minimal ductility. To enable further processing and to investigate the influence of silver and various heat treatments on the properties of bars composed of steel 03Kh17N10M2, annealing, normalization, and quenching of the resulting bars were conducted. Fig. 3 exhibits images of three alloys post-normalization at 900 °C, while Fig. 4 showcases the microstructure subsequent to annealing at 950 °C. Fig. 5 demonstrates the microstructure following quenching at 950 °C.
Fig. 3. Microstructure of the bars after normalization at 900 °C (holding time – 2 min):
Fig. 4. Microstructure of the bars after annealing at 950 °C (holding time – 2.5 min):
Fig. 5. Microstructure of the bars after quenching at 950 °C (holding time – 2.5 min): |
Following heat treatments, the wire materials undergo recrystallization, leading to the formation of a fine-grained structure with grain sizes ranging from 3 to 6 μm.
Upon quenching, an equiaxed and finely dispersed austenite structure becomes apparent. The presence of banding suggests that recrystallization was incomplete before the samples experienced accelerated cooling. Samples cooled in a furnace displayed grains with a more uniform shape than those cooled in water. Interestingly, annealed samples exhibited superior etchability when contrasted with quenched ones. Samples normalized at 900 °C exhibited similar microstructures to those subjected to the quenching process.
The microstructures of all compositions, regardless of the presence of silver, exhibit no significant differences from each other. This indicates that microdoping does not yield discernible microstructural changes.
Mechanical properties of steels from melts 1 to 3 were evaluated after undergoing various heat treatments, with a summary of the test results presented in Table 2.
Table 2. Mechanical properties of a bar with diameter of 2.4 mm, depending
|
Heat treatment of bars with a 2.4 mm diameter consistently results in a notable increase in ductility, which is essential for alleviating cold-working and producing wire of smaller diameters. Notably, the most pronounced effect was observed in the case of 03Kh17N10M2 without the addition of silver, wherein quenching facilitated achieving a relative elongation of over 50 %. The influence of silver on the mechanical properties was marginal, resulting in a slight reduction in ductility. Consequently, quenching was deemed the most suitable method for preparing the wire for further drawing to a 1 mm diameter.
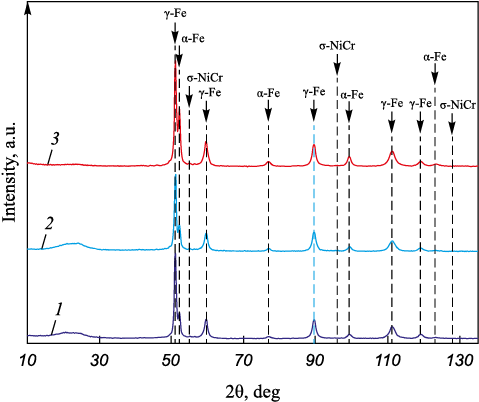
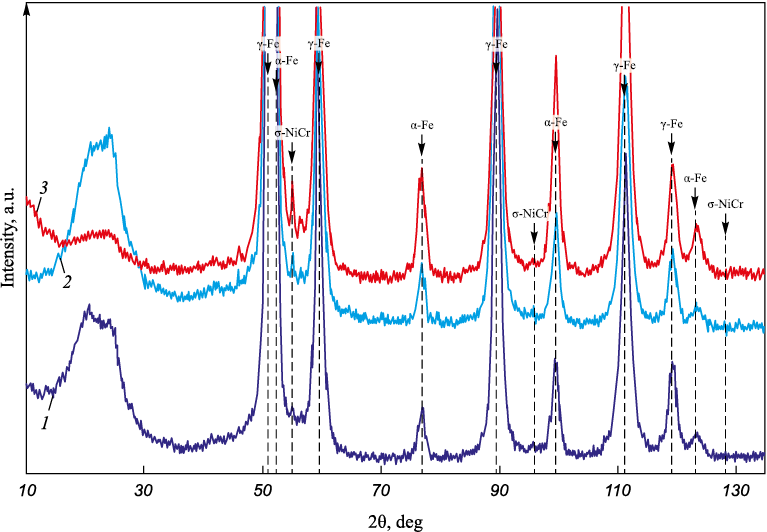
To delve deeper into the impact of silver, X-ray phase analysis was conducted on the wires with a 1 mm diameter. The phase composition data for the wires are outlined in Table 3 and depicted in Figs. 6, 7.
Table 3. Phase composition and parameters of the crystal lattice
Fig. 6. Diffractogram with the results of phase analysis
Fig. 7. Enlarged diffractogram with the results of phase analysis |
The analysis of the phase composition revealed a decrease in the γ-Fe fraction and an increase in α-Fe and σ-NiCr from wire composition 1 to composition 3. This denotes a ferrite-forming effect attributed to silver in the stainless steel composition. The escalation in silver content correlates with an increase in the α-Fe and σ-NiCr phases. The presence of ferrite results from significant plastic deformation during wire drawing, and it remains unaltered since the steel’s content of austenitizing elements (such as carbon, manganese, and nickel) is relatively low. Considering potential applications involving the produced wire in its current form, heat treatment might be advisable to achieve a single-phase structure.
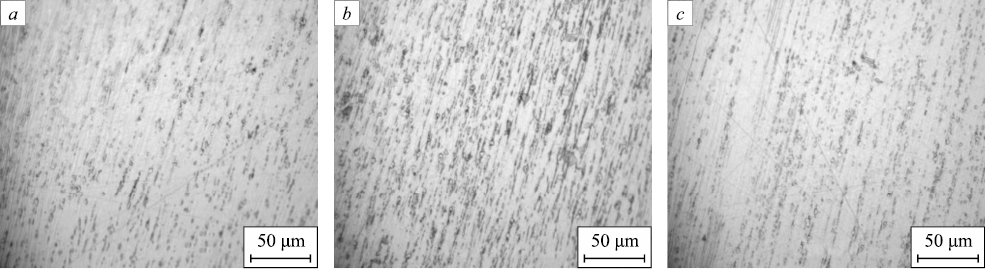
Fig. 8 displays the microstructure of the wires utilized in the phase analysis, highlighting the hardening effect post-drawing.
Fig. 8. Microstructure of the wires with a diameter of 1 mm obtained |
Table 4 presents the mechanical properties of the resulting wires from compositions 1 – 3 after being drawn to a 1 mm diameter.
Table 4. Mechanical properties of wires with diameter of 1 mm
|
Comparing the mechanical properties of the original composition wire with the alloyed compositions, it was observed that the wire with the addition of silver demonstrated similar mechanical characteristics.
Samples of strain-hardened wire with a 1 mm diameter were subjected to heat treatments at temperatures of 900, 950, 1000, 1050 °C, each for a duration of 2.5 min. The results of mechanical tests conducted on the material after these heat treatments are summarized in Table 5.
Table 5. Mechanical properties of the wire with composition 1 and diameter,
|
It has been observed that as the heating temperature for hardening increases, ductility also increases while strength decreases. This phenomenon occurs due to a reduction in the density of dislocations and an increase in grain size within the material. The choice of cooling medium (air or water) exhibits a similar effect on the mechanical properties owing to the relatively small diameter of the wire.
The acquired data aligns with established recommendations for the heat treatment of chromium-nickel-molybdenum steels. Furthermore, the results from mechanical tests conducted on bars, despite the inclusion of silver in the steel composition, do not demonstrate any anomalous findings. Notably, the observed ferrite-forming effect of silver, as identified through X-ray phase analysis, corresponds with findings in prior research [20]. In the mentioned study, the addition of 0.2 % Ag to 2205 DSS steel resulted in a 1.1 % increase in the ferrite phase content. However, in the case of steel 03Kh17N10M2, this effect was more than two times greater, measuring at 2.3 %. This disparity is likely due to the initial disparity in the ferrite phase content between 2205 DSS steel, which inherently had a significantly higher amount of ferrite phase, and 03Kh17N10M2 steel.
Conclusions
The investigation into the mechanical properties of wires made of austenitic stainless steel 03Kh17N10M2, both without and with silver additions of 0.2 and 0.5 %, revealed a slight reduction in ductility and an increase in strength due to silver doping. Furthermore, the escalation in silver content induced a shift in the phase composition, characterized by a decrease in the γ-phase and an increase in the α-phase and σ-phase. Specifically, the addition of 0.5 % Ag resulted in an 11.1 % decrease in the austenite fraction.
Post-heat treatments, irrespective of wire chemical composition and diameter, recrystallization occurred, fostering the development of a fine-grained structure with grain sizes ranging from 3 to 6 μm.
Interestingly, quenching the resulting 1 mm diameter wire in both air and water yielded similar outcomes. This suggests that for products made from steels with examined compositions, quenching up to a diameter of 1 mm can be effectively executed in air. However, when dealing with diameters larger than 2 mm, the type of heat treatment yields significant variations in mechanical properties.
References
1. Chen Q., Thouas G.A. Metallic implant biomaterials. Materials Science and Engineering: R: Reports. 2015;87:1–57. https://doi.org/10.1016/j.mser.2014.10.001
2. Kolmakov A.G., Ivannikov A.Yu., Kaplan M.A., Kirsankin A.A., Sevost’yanov M.A. Corrosion-resistant steels in additive manufacturing. Izvestiya. Ferrous Metallurgy. 2021;64(9):619–650. (In Russ.). https://doi.org/10.17073/0368-0797-2021-9-619-650
3. Kaplan M.A., Ivannikov A.Yu., Konushkin S.V., etс. Investigation of the structure, mechanical and antibacterial properties of corrosion-resistant steel alloyed with silver and titanium. Reports of the Russian Academy of Sciences. Chemistry, Materials Sciences. 2022;502(2):41–49. (In Russ.). https://doi.org/10.31857/S268695352201006X
4. State standard R 51148-98. Medical products. Requirements for samples and documentation submitted for toxicological, sanitary and chemical tests, sterility and pyrogenicity tests. Moscow: Izd-vo standartov; 05.05.1998:17. (In Russ.).
5. State standard 30208-94. Surgical instruments. Metal materials. Part 1: Stainless steel. Moscow: Izd-vo standartov; 01.10.2002:7. (In Russ.).
6. Zardiackas L.D. Stainless steels for implants. Wiley Encyclopedia of Biomedical Engineering. 2006:1–9. https://doi.org/10.1002/9780471740360.ebs1136
7. Dick J.C., Bourgeault C.A. Notch sensitivity of titanium alloy, commercially pure titanium, and stainless steel spinal implants. Spine. 2001;26(15):1668–1672. https://doi.org/10.1097/00007632-200108010-00008
8. Khosravi F., Nouri Khorasani S., Khalili S., etс. Development of a highly proliferated bilayer coating on 316L stainless steel implants. Polymers. 2020;12(5):1022. https://doi.org/10.3390/polym12051022
9. Rogers B.A., Little N.J. Surgical site infection with methicillin-resistant Staphylococcus aureus after primary total hip replacement. The Bone & Joint Journal. 2008;90-B(11): 1537–1538. https://doi.org/10.1302/0301-620X.90B11.21242
10. Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nature Reviews Microbiology. 2018;16(7):397–409. https://doi.org/10.1038/s41579-018-0019-y
11. Filipović U., Dahmane R.G., Ghannouchi S., Zore A., Bohinc K. Bacterial adhesion on orthopedic implants. Advances in Colloid and Interface Science. 2020;283:102228. https://doi.org/10.1016/j.cis.2020.102228
12. Arciola C.R., An Y.H., Campoccia D., Donati M.E., Montanaro L. Etiology of implant orthopedic infections: A survey on 1027 clinical isolates. The International Journal of Artificial Organs. 2005;28(11):1091–1100. https://doi.org/10.1177/039139880502801106
13. Rai M.K., Deshmukh S.D., Ingle A.P., Gade A.K. Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. Journal of Applied Microbiology. 2012;112(5):841–852. https://doi.org/10.1111/j.1365-2672.2012.05253.x
14. Morones J.R., Elechiguerra J.L., Camacho A., Holt K., Kouri J.B., Ramírez J.T., Yacaman M.J. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346. https://doi.org/10.1088/0957-4484/16/10/059
15. Baker C., Pradhan A., Pakstis L., Pochan D.J., Shah S.I. Synthesis and antibacterial properties of silver nanoparticles. Journal of Nanoscience and Nanotechnology. 2005;5(2): 244–249. https://doi.org/10.1166/JNN.2005.034
16. Yamanaka M., Hara K., Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Applied and Environmental Microbiology. 2005;71(11):7589–7593. https://doi.org/10.1128/AEM.71.11.7589-7593.2005
17. Mirzaee M., Vaezi M., Palizdar Y. Synthesis and characterization of silver doped hydroxyapatite nanocomposite coatings and evaluation of their antibacterial and corrosion resistance properties in simulated body fluid. Materials Science and Engineering: C. 2016;69:675–684. https://doi.org/10.1016/j.msec.2016.07.057
18. Gobi S.K., Sudhakar T., Karthik Al., etc. Silver-calcia stabilized zirconia nanocomposite coated medical grade stainless steel as potential bioimplants. Surfaces and Interfaces. 2021;24:101086. https://doi.org/10.1016/j.surfin.2021.101086
19. Kaplan M.A., Gorbenko A.D., Ivannikov A.Y., etc. Investigation of antibacterial properties of corrosion-resistant 316L steel alloyed with 0.2 wt.% and 0.5 wt.% Ag. Materials. 2023;16(1):319. https://doi.org/10.3390/ma16010319
20. Yang S.M., Chen Y.C., Pan Y.T., Lin D.Y. Effect of silver on microstructure and antibacterial property of 2205 duplex stainless steel. Materials Science and Engineering: C. 2016;63:376–383. https://doi.org/10.1016/j.msec.2016.03.014
21. Gong P., Li H., He X., Wang K., Hu J., Tan W., Zhang S., Yang X. Preparation and antibacterial activity of Fe3O4@Ag nanoparticles. Nanotechnology. 2007;18(28):285604. https://doi.org/10.1088/0957-4484/18/28/285604
About the Authors
A. D. GorbenkoRussian Federation
Artem D. Gorbenko, Research Engineer, Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences; Research Engineer, All-Russian Research Institute of Phytopathology
49 Leninskii Ave., Moscow 119991, Russian Federation
5 Institut Str., Bol’shie Vyazemy Vil., Odintsovo District, Moscow Region 143050, Russian Federation
M. A. Kaplan
Russian Federation
Mikhail A. Kaplan, Junior Researcher
49 Leninskii Ave., Moscow 119991, Russian Federation
S. V. Konushkin
Russian Federation
Sergei V. Konushkin, Junior Researcher
49 Leninskii Ave., Moscow 119991, Russian Federation
E. O. Nasakina
Russian Federation
Elena O. Nasakina, Senior Researcher
49 Leninskii Ave., Moscow 119991, Russian Federation
A. S. Baikin
Russian Federation
Aleksandr S. Baikin, Research Associate
49 Leninskii Ave., Moscow 119991, Russian Federation
K. V. Sergienko
Russian Federation
Konstantin V. Sergienko, Junior Researcher
49 Leninskii Ave., Moscow 119991, Russian Federation
A. Yu. Ivannikov
Russian Federation
Aleksandr Yu. Ivannikov, Cand. Sci. (Eng.), Senior Researcher
49 Leninskii Ave., Moscow 119991, Russian Federation
Ya. A. Morozova
Russian Federation
Yaroslava A. Morozova, Research Engineer, Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences; Research Engineer, All-Russian Research Institute of Phytopathology
49 Leninskii Ave., Moscow 119991, Russian Federation
5 Institut Str., Bol’shie Vyazemy Vil., Odintsovo District, Moscow Region 143050, Russian Federation
S. A. Oshkukov
Russian Federation
Sergei A. Oshkukov, Cand. Sci. (Medical), Senior Researcher
61/2 Shchepkina Str., Moscow 129110, Russian Federation
A. G. Kolmakov
Russian Federation
Aleksei G. Kolmakov, Corresponding Member of RAS, Dr. Sci. (Eng.), Head of the Laboratory
49 Leninskii Ave., Moscow 119991, Russian Federation
M. A. Sevost’yanov
Russian Federation
Mikhail A. Sevost’yanov, Cand. Sci. (Eng.), Leading Researcher, Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences; Head of the Center, All-Russian Research Institute of Phytopathology
49 Leninskii Ave., Moscow 119991, Russian Federation
5 Institut Str., Bol’shie Vyazemy Vil., Odintsovo District, Moscow Region 143050, Russian Federation
Review
For citations:
Gorbenko A.D., Kaplan M.A., Konushkin S.V., Nasakina E.O., Baikin A.S., Sergienko K.V., Ivannikov A.Yu., Morozova Ya.A., Oshkukov S.A., Kolmakov A.G., Sevost’yanov M.A. Effect of silver and heat treatment on properties of 03Kh17N10M2 austenitic steel wire. Izvestiya. Ferrous Metallurgy. 2023;66(5):544-553. https://doi.org/10.17073/0368-0797-2023-5-544-553
JATS XML