Scroll to:
Selective solid-phase reduction of iron in phosphorous oolite ores
https://doi.org/10.17073/0368-0797-2023-4-479-484
Abstract
Selective solid-phase reduction of iron and phosphorus in oolite ores of the Lisakovsky and Ayat deposits was experimentally studied. Using X-ray phase analysis, the phase composition of the initial ores and samples after reduction roasting was determined. Goethite, magnetite and quartz were found in the ores of both deposits. Phosphorus in the ore of the Ayat deposit is in the form of aluminum phosphate and iron hydrophosphate, and in the samples of the Lisakovsky ore – as a component of calcium hydrophosphate. Experiments on reduction roasting were carried out in a resistance furnace at 1000 °C with holding time of 5 h. After roasting in CO atmosphere, α-Fe appears in the samples, while phosphorus remains as a component of iron, calcium and aluminum phosphates. After roasting in a mixture with graphite, phosphorus is reduced by solid carbon from iron and calcium phosphates and passes into metal, but remains as a component of aluminum phosphate. Studies using microroentgenospectral analysis show that phosphorus content in the metal phase after reduction with solid carbon is 2.0 – 3.5 at. %. When CO is reduced in the atmosphere, phosphorus in the metallic phase is practically not detected. At the same time, the amount of residual iron in the oxide phase after carbon monoxide reduction significantly exceeds the amount of iron after reduction in a mixture with carbon. The experimental results confirm the possibility of selective reduction of iron by carbon oxide CO without phosphorus reduction.
Keywords
For citations:
Suleimen B., Salikhov S.P., Sharipov F.Sh., Roshchin V.E. Selective solid-phase reduction of iron in phosphorous oolite ores. Izvestiya. Ferrous Metallurgy. 2023;66(4):479-484. https://doi.org/10.17073/0368-0797-2023-4-479-484
Introduction
In light of the increased production of ferrous metals and the use of ores with low iron content, the integrated utilization of mineral raw materials in ferrous metallurgy has gained growing relevance [1 – 3]. In recent years, significant attention has been directed towards the challenges associated with the extraction [4 – 6] and processing [7] of iron from oolitic iron ores characterized by high phosphorus content. Additionally, considerable focus has been placed on the enrichment [8] and dephosphorization [9] of these ores. Extensive reserves of oolitic ores are present in Asian countries [10; 11], Africa [12; 13], Europe, and North America [13 – 15]. The Ayat and Lisakovsky deposits, situated in Kazakhstan, are examples of such deposits. Although these two large deposits share similar iron contents, they exhibit variations in phosphorus, vanadium, and aluminum content.
The unconsolidated fraction of Ayat ores comprises fragments of oolites, micrograins of quartz, and alumina, while the cohesive portion consists of oolites bound together by a clay-cement matrix [16]. The average iron content for this deposit is 37.1 %. The ores exhibit 16.4 % SiO2 , 6 % Al2O3 and 0.37 % P. Lisakovsky ores constitute a loose blend of brown iron ore oolites and quartz sand grains, featuring an iron content ranging from 30 to 40 % and a notably high phosphorus content (up to 0.8 %) [17]. The beneficiation of such ores necessitates the implementation of intricate and costly processing flowcharts. Unfortunately, the current methods do not effectively remove phosphorus, thereby impacting the technological aspects of metallurgical processing. Within the blast furnace, where roasting (in the shaft) and melting (in the hearth) occur in a reducing atmosphere, phosphorus undergoes complete reduction, transforming into metal. The elimination of phosphorus from cast iron, whether in ladles or steelmaking units, incurs additional expenditures in terms of materials, energy, and time. Consequently, dephosphorization before or during the iron production process emerges as a crucial step when utilizing high-phosphorus oolitic ores.
In [18 – 20], investigations were conducted into the feasibility of selectively reducing iron in the ores from the Ayat deposit through solid-phase reduction. According to the findings from these studies, selective iron reduction can be accomplished through exposure to carbon monoxide at a temperature of 1000 °С.
The objective of this study is to conduct a comparative analysis of the solid-phase selective reduction process of iron, without concurrent phosphorus reduction, in oolitic ores sourced from the Ayat and Lisakovsky deposits.
Experimental
The study utilized samples of oolitic iron ores obtained from the Ayat and Lisakovsky deposits. The experiments were conducted within a closed Tamman furnace featuring a graphite heater, ensuring the establishment of a reducing atmosphere in the furnace space. Employing the calculation method outlined in [21], the equilibrium composition of the gas phase in the furnace’s working space at a temperature of 1000 °C and a pressure of 0.1 MPa was determined to be 34.58 % CO, 0.07 % CO2 and 65.35 % N2 .
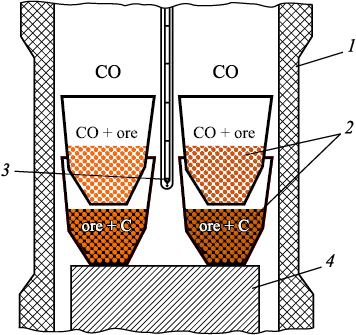
Four corundum crucibles (Fig. 1) were positioned in the working space of the furnace, each containing ore samples ranging in size from 0.4 to 1.0 mm. The ore samples in the upper crucibles interacted with CO oxide in the gas phase, while those in the lower crucibles were mixed with graphite powder and engaged with solid carbon. The furnace was gradually heated to a temperature of 1000 °C over 60 min and maintained at this temperature for a duration of 5 h. Temperature control was facilitated using a tungsten rhenium thermocouple WR5/WR20. The selection of temperature and holding time was based on insights gleaned from prior experiments [18 – 20].
Fig. 1. Layout of crucibles with ore samples in working space |
Upon completion of the holding period, the crucibles containing the samples were gradually cooled along with the furnace until reaching room temperature. To eliminate carbon residues, a mixture of samples with graphite powder was dispersed, and representative samples were extracted for X-ray spectral and X-ray phase microanalyses.
A subset of the samples was impregnated with epoxy resin, followed by grinding and polishing. The resultant polished sections were subjected to examination using a JSM-6460LV electron microscope (JEOL), equipped with an energy-dispersive analyzer (Oxford Instruments). This analysis aimed to ascertain the elemental composition at specific points and across areas through X-ray spectral microanalysis. X-ray phase analysis (XRF) of both the original and metallized samples was conducted using a Rigaku Ultima IV diffractometer. The resulting diffraction patterns were interpreted using the Match! 3 software.
Results
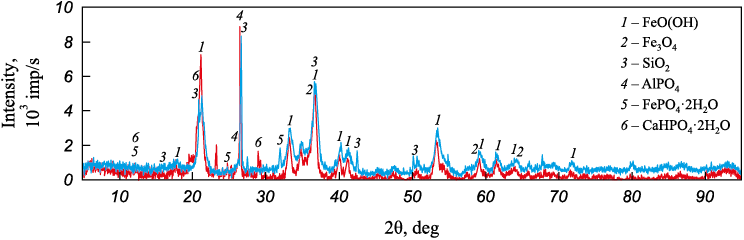
Fig. 2 presents the XRF results of the original ore samples, revealing the presence of goethite FeO(OH), magnetite Fe3O4 and quartz SiO2 in both samples. The Ayat deposit ore contains phosphorus in the form of aluminum phosphate AlPO4 and iron hydrogen phosphate FePO4∙2H2O. In contrast, the Lisakovsky ore samples include phosphorus in the composition of calcium hydrogen phosphate CaHPO4·2H2O.
Fig. 2. Diffractograms of samples of initial ores from Lisakovsky ( |
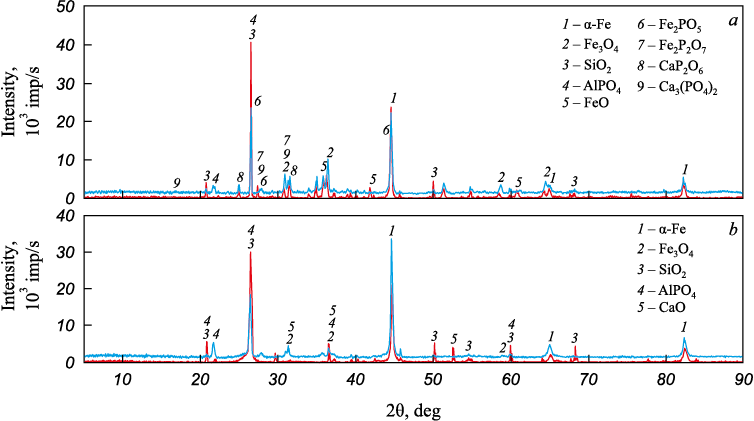
Fig. 3 displays the XRF results after reduction roasting. The diffraction pattern of samples reduced in a CO oxide atmosphere (Fig. 3, a) exhibits a higher number of peaks, indicating a greater variety of phases compared to samples roasted in contact with graphite powder (Fig. 3, b).
Fig. 3. Diffractogram of the ores from Lisakovsky ( |
According to the XRF results, all samples contain α-iron, magnetite Fe3O4 , quartz SiO2 and berlinite AlPO4 . In samples subjected to CO atmosphere, phosphorus manifests itself in the composition of iron and calcium phosphates, namely FeP2O7 and FePO4 , CaP2O6 and Ca3(PO4)2 (Fig. 3, a). Conversely, samples in contact with carbon do not contain iron or calcium phosphates. The Lisakovsky ore exhibits the presence of calcium oxide CaO (Fig. 3, b).
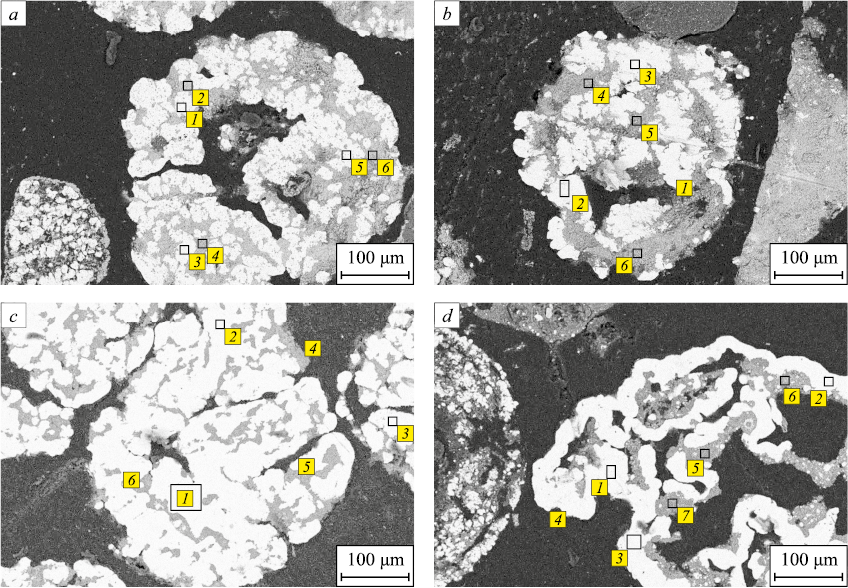
An examination of polished sections of roasted ores revealed that, whether in contact with carbon or in a CO atmosphere, the metallic phase of iron formed both on the surface and within the ore particles (Fig. 4). Notably, reduction with solid carbon resulted in the creation of more distinctly defined and dense metallic structures (Fig. 4, d).
Fig. 4. Distribution of metallic and non-metallic phases in the samples of ores of Lisakovsky (a, c) |
The table provides the average results of X-ray spectral microanalysis, indicating the elemental content at specific points and sites of analysis in the metallized ore samples. As an illustration, based on the analysis results from sites 1, 3 and 5 (Fig. 4, a), the average phosphorus content is 0.1 %, while the iron content is 99.9 % (at.).
Average content of elements according to results of the metallized samples analysis
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Post-roasting in contact with carbon, iron in samples from both deposits undergoes nearly complete reduction, with residual oxides containing only 5 – 7 % iron. Conversely, when reduced in a CO atmosphere, the iron content in the oxide phase remains around 20 %. Notably, in iron reduced in a CO atmosphere, the phosphorus content in the metal does not exceed 0.1 %. In contrast, after the reduction of iron with solid carbon, the phosphorus content ranges between 2.5 – 3.0 %.
Discussion
The XRF results indicate that the initial ores comprise phases of goethite, magnetite, hydrophosphates of iron and calcium, quartz, and aluminum phosphate. In all metallized samples, goethite disappears, the α-iron phase emerges, and the SiO2 phase persists. Phosphorus in samples after reduction in a CO atmosphere is found in the form of CaP2O6 or Ca3(PO4)2 , FePO4 or FeP2O7 and AlPO4 . In contrast, samples reduced with carbon show phosphorus exclusively in the AlPO4 phase. Under these conditions, phosphorus undergoes reduction from calcium and iron phosphates, converting into metal. The obtained results are confirmed by the examination of the samples using an electron microscope after reduction roasting. In the Ayat and Lisakovsky deposit samples, phosphorus is virtually not reduced after reduction in a CO atmosphere; however, in a mixture with solid carbon, phosphorus reduction occurs and is detectable through X-ray spectral microanalysis in the metal phase.
The increased phosphorus content in Lisakovsky ore does not alter the previously identified patterns of its recovery but rather reinforces the obtained findings. Thus, carbon monoxide does not reduce phosphorus from oolitic ore compounds, while phosphorus reduction is achieved through the utilization of solid carbon.
The results obtained affirm the feasibility of selectively reducing iron with carbon monoxide in oolitic ores characterized by high phosphorus content from different deposits.
Conclusions
Lisakovsky and Ayat oolitic ores share a similarity in iron content but exhibit variations in phosphorus content, where phosphorus is present in the form of calcium, iron, and aluminum phosphates. At a temperature of 1000 °C and with a holding time of 5 h, carbon monoxide fails to reduce phosphorus from iron and calcium hydrophosphates, as well as from aluminum phosphates. In contrast, under the same conditions, when in contact with solid carbon, phosphorus undergoes complete reduction, transitioning into the metal phase from calcium and iron hydrophosphates. However, reduction from aluminum phosphate does not occur.
References
1. Smirnov K.I., Gamov P.A. Pyro-metallurgical processing of ilmenite concentrate with production of iron and titanium oxides. Solid State Phenomena. 2021;316:385–389. https://doi.org/10.4028/www.scientific.net/SSP.316.385
2. Smirnov K., Gamov P.A. Specific features of metal reduction from ilmenite concentrate. AIP Conference Proceedings. 2022;2456(1):020052. https://doi.org/10.1063/5.0074718
3. Kosdauletov N.K., Roshchin V.R. Solid-phase reduction and separation of iron and phosphorus from manganese oxides in ferromanganese ore. Defect and Diffusion Forum. 2021;410:281–286. https://doi.org/10.4028/www.scientific.net/DDF.410.281
4. Li K., Ni W., Zhu M., Zheng M., Li Y. Iron extraction from oolitic iron ore by a deep reduction process. Journal of Iron and Steel Research International. 2011;18(8):9–13. http://doi.org/10.1016/S1006-706X(11)60096-4
5. Sun Y.S., Han Y.X., Gao P., Wang Z.H., Ren D.Z. Recovery of iron from high phosphorus oolitic iron ore using coal-based reduction followed by magnetic separation. International Journal of Minerals, Metallurgy, and Materials. 2013;20(5):411–419. https://doi.org/10.1007/s12613-013-0744-1
6. Zhou W., Han Y., Sun Y., Gao P., Li Y. Review of research on iron extraction and phosphorus reduction of high phosphorus oolitic hematite. Metal Mines. 2019;(2):10–11. https://doi.org/10.19614/j.cnki.jsks.201902002
7. Zhou W., Han Y., Sun Y., Gao P., Li Y. Recycling iron from oolitic hematite via microwave fluidization roasting and magnetic separation. Minerals Engineering. 2021;164:106851. https://doi.org/10.1016/j.mineng.2021.106851
8. Chandio A.D., Channa I.A., Shaik A.A., Madad S., Rizvi S.B.H., Shah A.A., Alhazaa A. Beneficiation of low-grade Dilband iron ore by reduction roasting. Metals. 2023;13(2):296. https://doi.org/10.3390/met13020296
9. Wu S., Sun T., Kou J., Xu H. A new iron recovery and dephosphorization approach from highphosphorus oolitic iron ore via oxidation roasting-gas-based reduction and magnetic separation process. Powder Technology. 2023;413:118043. https://doi.org/10.1016/j.powtec.2022.118043
10. Manieh A.A. Oolite liberation of oolitic iron ore, Wadi Fatima, Saudi Arabia. International Journal of Mineral Processing. 1984;13(3):187–192. https://doi.org/10.1016/0301-7516(84)90002-4
11. Abro M.M., Pathan A.G., Mallah A.H. Liberation of oolitic hematite grains from iron ore. Mehran University Research, Journal of Engineering Technology. 2011;30:329–338.
12. El Sharkawi M.M., Mesaed A., Mortada M.E. Stratigraphic Setting and Paleoenvironment of the Coniacian-Santonian Ironstones of Aswan, South Egypt. 1996.
13. Champetier Y., Hamdadou E., Hamdadou M. Examples of biogenic support of mineralization in two oolitic iron ores-Lorraine (France) and Gara Djebilet (Algeria). Sedimentary Geology.1987;51(3-4):249–255. https://doi.org/10.1016/0037-0738(87)90050-9
14. Tigunov L.P., Anufrieva S.I., Bronitskaya E.S., Krivokoneva G.K., Sokolova V.N., Alikberov V.M., Sladkova G.A., Fainshtein G.G., Parovinchak M.S. Modern technological decisions of processing ferriferous ores of the Bakcharsky deposit. Razvedka i okhrana nedr. 2010;(2):37–43. (In Russ.).
15. Özdemir Ö., Deutsch E.R. Magnetic properties of oolitic iron ore on Bell Island, Newfoundland. Earth and Planetary Science Letters. 1984;69(2):427–441. https://doi.org/10.1016/0012-821X(84)90201-2
16. Suleimen B., Salikhov S.P., Roshchin V.E. Study of the iron ores of the Ayat deposit of the oolite-type. Mining Informational and Analytical Bulletin (Scientific and Technical Journal). 2022;(10-1):50–58. (In Russ.). https://doi.org/10.25018/0236_1493_2022_101_0_50
17. Kaskataeva K.B., Kryazheva T.V., Sadchikov A.V., D’yakonov V.V. Characteristics of ores of the Lisakovsky deposit for their complex processing. Izvestiya Tomskogo politekhnicheskogo universiteta. Inzhiniring georesursov. 2021;332(5):7–16. (In Russ.).
18. Salikhov S.P., Suleimen B., Roshchin V.E. Selective reduction of iron and phosphorus from oolite ore. Izvestiya. Ferrous Metallurgy. 2020;63(7):560–567. (In Russ.). https://doi.org/10.17073/0368-0797-2020-7-560-567
19. Suleimen B., Salikhov S.P. Behavior of extrusion briquettes (Brex) and pellets from oolite iron ore in solid-phase metallization. AIP Conference Proceedings. 2022;2456(1):020054. https://doi.org/10.1063/5.0075188
20. Suleimen B., Salikhov S.P. Metallization of oolitic iron ore after oxidation firing. Solid State Phenomena. 2021;316:390–395. https://doi.org/10.4028/www.scientific.net/SSP.316.390
21. Mikhailov G.G., Leonovich B.I., Kuznetsov Yu.S. Thermodynamics of Metallurgical Processes and Systems. Moscow: MISIS; 2009:520. (In Russ.).
About the Authors
B. SuleimenRussian Federation
Bakyt Suleimen, Research Associate of the Research Laboratory “Hydrogen Technologies in Metallurgy”
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
S. P. Salikhov
Russian Federation
Semen P. Salikhov, Cand. Sci. (Eng.), Assist. Prof. of the Chair of Pyrometallurgical and Foundry Technologies
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
F. Sh. Sharipov
Russian Federation
Farkhod Sh. Sharipov, MA Student of the Chair of Pyrometallurgical and Foundry Technologies
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
V. E. Roshchin
Russian Federation
Vasilii E. Roshchin, Dr. Sci. (Eng.), Prof. of the Chair of Pyrometallurgical and Foundry Technologies
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
Review
For citations:
Suleimen B., Salikhov S.P., Sharipov F.Sh., Roshchin V.E. Selective solid-phase reduction of iron in phosphorous oolite ores. Izvestiya. Ferrous Metallurgy. 2023;66(4):479-484. https://doi.org/10.17073/0368-0797-2023-4-479-484
JATS XML