Scroll to:
Physical properties and structure of boron-containing slags during reduction period of AOD process
https://doi.org/10.17073/0368-0797-2023-4-471-478
Abstract
The effect of basicity and content of boron oxide on viscosity, crystallization temperature, phase composition, and structure of the
СаО – SiO2 – B2O3 – 12 % Cr2O3 – 3 % Аl2O3 – 8 % МgO fluorine-free slag system in the range of boron oxide content 3 – 6 % and basicity 1.0 – 2.5 is studied by vibrational viscometry, thermodynamic phase composition modeling (HSC Chemistry 6.12 (Outokumpu)), and Raman spectroscopy. It was found that physical properties of the studied slags mainly depend on the balance between the degree of structure polymerization, nature of the bond with it, and phase composition. With a low basicity of 1.0, slags are “long” and an increase in the content of boron oxide from 3 to 6 % makes them more fusible, reducing the crystallization temperature of the slag from 1340 to 1224 °C, and its viscosity from 1.0 – 0.8 to ~0.25 Pa·s at 1600 – 1660 °C, despite the significant complication of the structure, reflected in the growth of the bridging oxygen index BO from 1.10 to 1.49. With an increase in basicity, slags transfer from “long” to “short” and the content of calcium oxide increases, which, being a donor of free oxygen ions (O2–), acts as a modifier of the slag structure. Thus, with a basicity of B = (CaO/SiO2) = 2.5, slags have a simpler structure (BO = 0.50 – 0.53) relative to slags with a basicity of 1.0, while the addition of boron oxide complicates it only slightly (an increase in BO from 0.5 up to 0.53). Increasing the concentration of B2O3 lowers the crystallization temperature from 1674 to 1605 °C and the viscosity from 1.0 to 0.3 Pa·s at 1660 °C as a result of the formation of low-melting compounds (mostly 2CaO·B2O3).
Keywords
For citations:
Shartdinov R.R., Babenko A.A., Upolovnikova A.G., Smetannikov A.N. Physical properties and structure of boron-containing slags during reduction period of AOD process. Izvestiya. Ferrous Metallurgy. 2023;66(4):471-478. https://doi.org/10.17073/0368-0797-2023-4-471-478
Introduction
Currently, the primary method employed for the smelting of low-carbon stainless steel is the argon oxygen decarburization (AOD). This technology, originated by Union Carbide Corp. in the USA in 1968, became widely adopted, and by the onset of the 21st century, approximately three-quarters of all stainless steel production used this technique [1].
The AOD process consists of two distinct phases: oxidation and reduction. The primary objective of the oxidation period is to decarbonize the metal by introducing a mixture of oxygen and inert gas, thereby attaining the required carbon concentrations while minimizing the oxidation of chromium. Subsequently, the reduction period ensues, during which the metal is purged solely with inert gas to enhance mixing and reintegrate oxidized chromium into the metal, accomplished through the addition of aluminum or silicon additives. Concluding the reduction phase, the metal undergoes desulfurization, resulting in the formation of slag characterized by low FeO oxide content and a basicity ranging from 2.0 to 2.5 [1]. Nevertheless, the effectiveness of achieving profound metal desulfurization and the efficient reduction of chromium is not solely dependent on the chemical activity of the oxide system components; it also relies on creating favorable kinetic conditions for the processes [1 – 3].
The kinetics of the processes involved in metal desulfurization and chromium reduction are predominantly influenced by the fluid mobility of the generated slags [1; 4]. The diffusion rates of sulfur and chromium oxide within the slag are inversely proportional to its viscosity [2]. To promote low viscosity in the resultant slag, fluorspar is frequently employed as a flux [1; 5; 6]. However, the utilization of the CaF2 compound poses a significant drawback due to the generation of environmentally harmful volatile fluorides at elevated temperatures during the process [3; 7]. The advancement of this process is accompanied by a reduction in the refining properties of the resulting slags, an escalation in environmental impact, and a corrosive effect on equipment. Therefore, there is a need to develop refining slags with enhanced fluid mobility that do not incorporate fluorspar. A viable solution to this challenge is the incorporation of boron oxide, which, through interactions with the primary components of the generated slags, forms low melting eutectics (CaO∙B2O3 and 2CaO∙B2O3 with melting points of 1130 and 1280 °C), ensuring heightened fluid mobility.
In this study, the viscosity (η), crystallization temperature (tcr), phase composition, and structure of slags in the СаО – SiO2 – B2O3 – 12 % Cr2O3 – 3 % Аl2O3 – 8 % МgO system were investigated across a range of boron oxide content from 3 to 6 % and basicity levels of 1.0 – 2.5. This investigation employed vibration viscometry, thermodynamic simulation of phase composition (HSC Chemistry 6.12 (Outokumpu)), and Raman spectroscopy.
Materials and experimental methods
In order to investigate the properties of slags within the СаО – SiO2 – B2O3 – 12 % Cr2O3 – 3 % Аl2O3 – 8 % МgO system, slags were prepared, and their composition outlined in Table 1.
Тable 1. Composition of the experimental slags
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
The slag was melted in a resistance furnace using molybdenum crucibles in an argon atmosphere, with oxides of analytical grade calcined for 2 – 3 h at a temperature of 800 °C (oxide B2O3 at 100 °C).
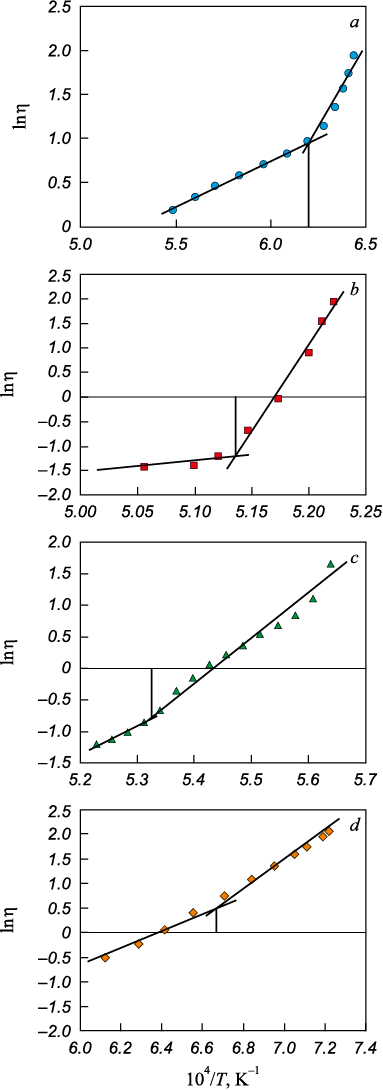
Viscosity measurements of the resulting slags were conducted employing a vibration viscometer [8] in molybdenum crucibles under an argon atmosphere. Temperature measurement was executed using a tungsten–rhenium thermocouple. The data obtained, characterizing slag viscosity as a function of temperature, were utilized to construct graphs in ln η – 1/T coordinates. The inflection point of the viscosity polytherms in these coordinates, following Frenkel’s theory of viscous flow, indicates the temperature at which slag crystallization initiates [9].
Thermodynamic simulation of the phase composition of experimental slag samples was performed using the HSC Chemistry 6.12 software package (Outokumpu) [10].
The structure of experimental slag samples was examined using a U 1000 Raman microscope-spectrometer with a laser featuring an exciting wavelength of 532 nm. The resulting spectra are presented graphically within the wavenumber range of 400 – 1500 cm\(^ - \)1.
Results and discussion
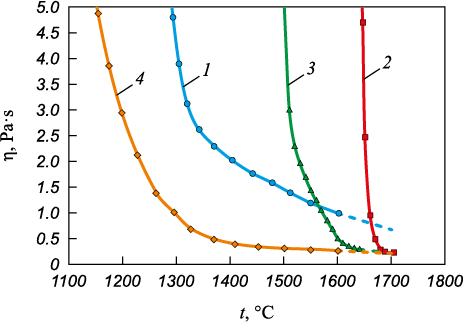
The measured viscosity of the slags within the oxide system under investigation is illustrated in Fig. 1, and in Fig. 2, they are depicted in ln η – 1/T coordinates; the onset temperature of crystallization was determined based on the inflection in the dependency (Table 1).
Fig. 1. Dependence of slag viscosity (1 – 4) on temperature
Fig. 2. Dependence of the logarithm of viscosity (ln η) |
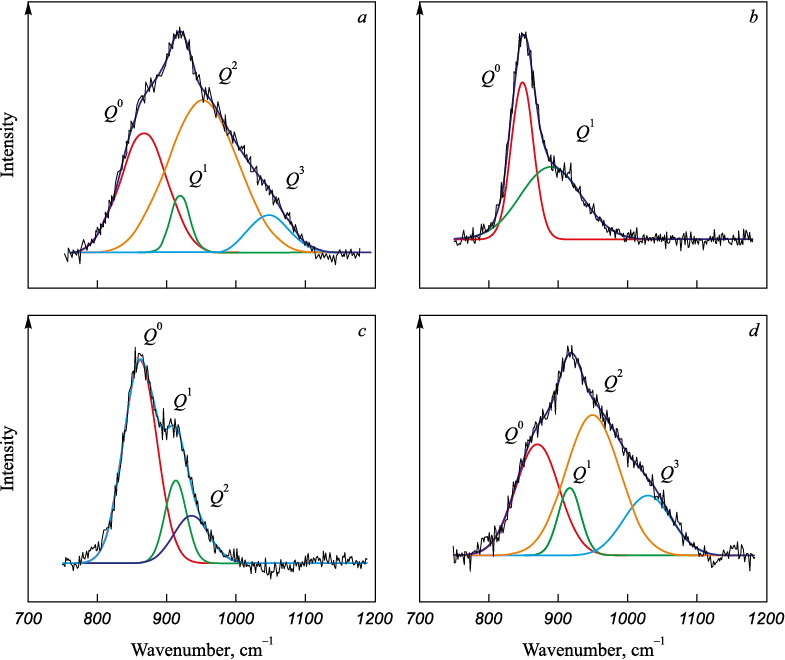
Throughout the study, Raman spectra of the examined slags 1 – 4 were acquired (Fig. 3).
Fig. 3. Raman spectra of the slag samples 1 (В = 1.0; 3 % В2О3), |
It is postulated that the extent of slag polymerization is primarily influenced by the high-frequency silicate range of 800 – 1200 cm\(^ - \)1, corresponding to [SiO4] tetrahedra. For a comprehensive understanding of the slag structure, deconvolution of the Raman spectra within this range was conducted using the Gaussian method [11]. The distinctive peaks of elements \(Q_{{\rm{Si}}}^n\) ([SiO4] with the number of bridging oxygen n) and others are detailed in Table 2, with the results of the deconvolution presented in Fig. 4.
Fig. 4. Deconvolated spectra for the slags 1 – 4 (a – d)
Table 2. Correspondence of wave numbers and structures
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A plausible method for representing the degree of slag polymerization is through the average quantity of bridging oxygen (BO). This metric is articulated as the number of bridging oxygen atoms multiplied by the relative fraction of each structural unit [SiO4] and is calculated using the formula outlined in Table 3:
BO = 0·\(Q_{{\rm{Si}}}^0\) + 1·\(Q_{{\rm{Si}}}^1\) + 2·\(Q_{{\rm{Si}}}^2\) + 3·\(Q_{{\rm{Si}}}^3\) + 4·\(Q_{{\rm{Si}}}^4\).
Table 3. Fractions of silicate structural elements
| |||||||||||||||||||||||||||||||||||||
Table 4 showcases the outcomes of the thermodynamic simulation of the phase composition of experimental slag samples. The results, determined by the melting temperatures of the formed phases, were categorically divided into three groups: low temperature (1130 – 1280 °C), medium temperature (1460 – 1600 °C), and high temperature (1710 – 2852 °C) phases.
Table 4. Phase composition of the experimental slags at 1650 °C
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Acidic slags with a basicity of 1.0 (samples 1 and 4) fall into the category of “long” slags (Fig. 1), characterized by a high degree of polymerization (Table 3). Fig. 3 does not display peaks corresponding to the [BO3] compound. It can be inferred that the B2O3 oxide is incorporated into the structure through 3D tetrahedra of the [BO4 ] compound, aligning with wave numbers of 900 – 920 cm\(^ - \)1 (Table 2).
As per the deconvolution results, slag 1, with a basicity of 1.0 and 3 % B2O3 , possesses a BO index value of 1.1. Its structure is predominantly represented by the [SiO4] compound without bridging oxygen, along with 1 and 2 bridging oxygen, with proportions of 0.39, 0.17 and 0.41, respectively. The combination of a relatively complex silicon–oxygen network structure (BO = 1.1) with a high concentration of high-temperature phases (32.23 %) leads to the formation of slag characterized by high viscosity of 1.0 – 0.8 Pa∙s at 1600 – 1660 °C and a crystallization temperature of 1340 °C (Table 1).
Increasing the boron oxide content to 6 % induces even greater polymerization in slag 4 (BO = 1.49). This leads to an elevated proportion of Q2 (0.45) and Q3 (0.17), primarily due to changes in Q\(^ 0 \) and Q1 values. At the same time, the proportion of low-melting compounds in the slag rises to 26.3 %, while the content of high-temperature phases decreases to 30.66 %. Despite the presence of a more intricate silicon-oxygen structure (BO = 1.49), in the form of [BO4 ] tetrahedra, weakens the complex silicon-oxygen lattice. This weakening occurs as the resulting B – O\(^ 0 \) bonds are weaker than the Si – O\(^ 0 \) bonds. This “weakening” of the slag structure, combined with an increase in the proportion of low-melting compounds, results in a reduced viscosity of slag 4 to approximately 0.25 Pa∙s at a temperature of 1600 – 1660 °С.
Slags 2 and 3, characterized by high basicity of 2.5, exhibit a “shorter” nature with a low degree of polymerization (refer to Fig. 1 and Table 3). As the basicity of these slags increases to 2.5, the peak in the silicate region of the spectrum (800 – 1200 cm\(^ - \)1 shifts towards a decrease in wavenumber (Fig. 3). This shift is attributed to calcium oxide (CaO), acting as a slag structure modifier by providing free oxygen ions (O2\(^ - \)). These free oxygen ions react with bridging oxygen (O\(^ 0 \)) in silicates, resulting in a reduction in the complexity of Si–O bonds in the slag structure. Consequently, an increase in CaO content promotes the development of the depolymerization process [19 – 23]. Peaks in the wavenumber range of 500 – 650 cm\(^ - \)1 correspond to the Cr – O – Cr, Si – O – Si and Al – O – Al bonds. With an increase in basicity, these peaks smooth out, indicating a weakening of the bonds.
Slag 2, containing 3 % boron oxide, possesses the least complex structure (BO = 0.5). It is characterized by an equal number of Q\(^ 0 \) and Q1 values featuring a simple silicon-oxygen structure with a small amount of bridging oxygen. The slag is distinguished by a high proportion of refractory phases (more than 65 %) and a small proportion of low-melting phases (2.94 %). Consequently, its crystallization temperature is 1676 °C, and viscosity is 1.0 Pa∙s at a temperature of 1660 °С.
Increasing the boron oxide content to 6 % in slag 3 has virtually no effect on its polymerization compared to slag 2 (the amount of bridging oxygen does not exceed 0.53). The structure contains Q\(^ 0 \), Q1 and Q2, with proportion of 0.63, 0.21 and 0.16, respectively. However, an increase in the content of low-melting phases to 8.05 % and a decrease in the proportion of refractory phases to 53.86 % positively influence the crystallization temperature (1605 °C) and slag viscosity, which decreased to 0.5 – 0.3 Pa∙s in the range of 1600 – 1660 °С.
The obtained data on the influence of slag basicity and boron oxide content on phase composition, structure, viscosity, and crystallization temperature highlight that slag viscosity depends on the balance between the degree of polymerization of the structure, the nature of the bonds within it, and the phase composition.
Conclusions
The study has yielded new data on the impact of basicity and boron oxide content on the viscosity, crystallization temperature, phase composition, and structure of slags within the СаО – SiO2 – B2O3 – 12 % Cr2O3 – 3 % Аl2O3 – 8 % МgO system, spanning a range of boron oxide content from 3 to 6 % and basicity from 1.0 to 2.5.
The findings reveal that the physical properties of the investigated slags predominantly hinge on the delicate equilibrium between the degree of polymerization of the structure, the nature of the bonds within it, and the phase composition. At a low basicity of 1.0, augmenting the boron oxide content from 3 to 6 % renders the slag more fusible, leading to a reduction in the crystallization temperature from 1340 to 1224 °C and a decrease in viscosity from 1.0 – 0.8 to approximately 0.25 Pa∙s at a temperature of 1600 – 1660 °C. This occurs despite a notable increase in structural complexity, as reflected in the rise of the BO index from 1.10 to 1.49.
In the case of high basicity (B = 2.5), the slags exhibit a simpler structure (BO = 0.50 – 0.53), and the addition of boron oxide only marginally complicates it (from 0.50 to 0.53). An increase in B2O3 content results in a reduction of the crystallization temperature from 1674 to 1605 °C and a decrease in viscosity from 1.0 to 0.3 Pa∙s at a temperature of 1660 °C, attributed to the formation of low-melting compounds.
References
1. Tokovoi O.K. Argon Oxygen Refining of Stainless Steel. Chelyabinsk: ITs YuUrGU; 2015:250. (In Russ.).
2. Popel’ S.I., Sotnikov A.I., Boronenkov V.N. Theory of Metallurgical Processes. Moscow: Metallurgiya; 1986:463. (In Russ.).
3. Dyudkin D.A., Kisilenko V.V. Production of Steel. In 3 vols. Vol. 3. Out-of-Furnace Steel Metallurgy. Moscow: Teplotekhnik; 2010:544. (In Russ.).
4. Yavoiskii V.I., Yavoiskii A.V. Scientific Foundations of Modern Production Processes of Steel. Moscow: Metallurgiya; 1987:184. (In Russ.).
5. Magidson I.A., Morozov A.S., Sidorenko M.F., etc. Viscosity of chromium slags. Izvestiya. Ferrous Metallurgy.1973;16(11):61–64. (In Russ.).
6. Kalicka Z., Kawecka-Cebula E., Pytel K. Application of the Iida model for estimation of slag viscosity for Al2O3–Cr2O3–CaO–CaF2 systems. Archives of Metallurgy and Materials. 2009;54(1):179–187.
7. Povolotskii D.Ya., Roshchin V.E., Gribanov V.P., etc. Influence of SiO2 on the volatility of slags of Al2O3–Al2O3–CaF2 system. Izvestiya. Ferrous metallurgy. 1982;25(8):39–42. (In Russ.).
8. Shtengel’meier S.V., Prusov V.A., Bogechov V.A. Improving viscosity measuring with a vibration viscometer. Zavodskaya laboratoriya. 1985;(9):56–57. (In Russ.).
9. Voskoboinikov V.G., etc. Properties of Blast Furnace Slags. Moscow: Metallurgiya; 1975:180. (In Russ.)..
10. Roine A. HSC 6.0 Chemistry Reactions and Equilibrium Software with Extensive Thermochemical Database and Flowshut. Pori.: Outokumpu research Oy; 2006:448.
11. Mysen B.O., Virgo D., Scarfe C.M. Relations between the anionic structure and viscosity of silicate melts-a Raman spectroscopic study. American Mineralogist. 1980;65(7): 690–710.
12. McMillan P. Structural studies of silicate glasses and melts-applications and limitations of Raman spectroscopy. American Mineralogist. 1984;69(6):622–644.
13. Matson D.W., Sharma S.K., Philpotts J.A. The structure of high-silica alkali-silicate glasses. A Raman spectroscopic investigation. Journal of Non-Crystalline Solids. 1983;58(2-3):
14. –352. https://doi.org/10.1016/0022-3093(83)90032-7
15. McMillan P.F., Poe B.T., Gillet P.H., Reynard B. A study of SiO2 glass and supercooled liquid to 1950 K via high-temperature Raman spectroscopy. Geochimica et Cosmochimica Acta. 2001;58(17):3653–3662. https://doi.org/10.1016/0016-7037(94)90156-2
16. Kim T.S., Park J.H. Structure-viscosity relationship of low-silica calcium aluminosilicate melts. ISIJ International. 2014;54(9):2031–2038. https://doi.org/10.2355/isijinternational.54.2031
17. Dines T.J., Inglis S. Raman spectroscopic study of supported chromium (VI) oxide catalysts. Physical Chemistry Chemical Physics. 2003;5(6):1320–1328. https://doi.org/10.1039/b211857b
18. Kim Y., Morita K. Relationship between molten oxide structure and thermal conductivity in the CaO–SiO2–B2O3 system. ISIJ International. 2014;54(9):2077–2083. https://doi.org/10.2355/isijinternational.54.2077
19. Cochain B., Neuville D.R., Henderson G.S., McCammon C.A., Pinet O., Richet P. Effects of the iron content and redox state on the structure of sodium borosilicate glasses: A Raman, Mössbauer and boron K‐Edge XANES spectroscopy study. Journal of the American Ceramic Society. 2012;95(3):962–971. https://doi.org/10.1111/j.1551-2916.2011.05020.x
20. Mysen B.O., Virgo D., Seifert F.A. The structure of silicate melts: Implications for chemical and physical properties of natural magma. Reviews of Geophysics. 1982;20(3):
21. –382. https://doi.org/10.1029/RG020i003p00353
22. Mysen B.O. Relationships between silicate melt structure and petrologic processes. Earth-Science Reviews. 1990;27(4): 281–365. https://doi.org/10.1016/0012-8252(90)90055-Z
23. Mills K.C. The influence of structure on the physico-chemical properties of slags. ISIJ International. 1993;33(1):148–155. https://doi.org/10.2355/isijinternational.33.148
24. Park J.H. Structure–property correlations of CaO–SiO2–MnO slag derived from Raman spectroscopy. ISIJ International. 2012;52(9):1627–1636. https://doi.org/10.2355/isijinternational.52.1627
25. Park J.H. Composition-structure-property relationships of CaO–MO–SiO2 (M = Mg2+, Mn2+) systems derived from micro-Raman spectroscopy. Journal of Non-Crystalline Solids. 2012;358(23):3096–3012. https://doi.org/10.1016/j.jnoncrysol.2012.08.014
About the Authors
R. R. ShartdinovRussian Federation
Ruslan R. Shartdinov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. A. Babenko
Russian Federation
Anatolii A. Babenko, Dr. Sci. (Eng.), Prof., Chief Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. G. Upolovnikova
Russian Federation
Alena G. Upolovnikova, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
A. N. Smetannikov
Russian Federation
Artem N. Smetannikov, Junior Researcher of the Laboratory of Steel and Ferroalloys
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Shartdinov R.R., Babenko A.A., Upolovnikova A.G., Smetannikov A.N. Physical properties and structure of boron-containing slags during reduction period of AOD process. Izvestiya. Ferrous Metallurgy. 2023;66(4):471-478. https://doi.org/10.17073/0368-0797-2023-4-471-478