Scroll to:
Carbides of transition metals: Properties, application and production. Review. Part 2. Chromium and zirconium carbides
https://doi.org/10.17073/0368-0797-2023-4-445-458
Abstract
The properties, application, and methods for producing chromium and zirconium carbides are considered. These carbides are oxygen-free refractory metal-like compounds. As a result, they are characterized by high values of thermal and electrical conductivity. Their hardness is relatively high. Chromium and zirconium carbides exhibit significant chemical resistance in aggressive environments. For these reasons, they have found application in modern technology. Chromium carbide is used mainly as component of surfacing mixtures to create protective coatings that resist intensive abrasive wear, including at elevated temperatures (up to 800 °C) in oxidizing environments. This compound is also used in the manufacture of tungsten-free hard alloys and carbide steels. Chromium carbide, along with vanadium carbide, is used as a grain growth inhibitor in WC – Co hard alloys. Powdered zirconium carbide can be used to polish the surface of items made of ferrous and non-ferrous metals. The properties of refractory compounds depend on the content of impurities and dispersion (particle size). To solve a specific problem associated with the use of refractory compounds, it is important to choose the right method for their preparation, to determine the permissible content of impurities in the initial components. This leads to the existence of different methods for the synthesis of carbides. The main methods for their preparation are: synthesis from simple substances (metals and carbon), metallothermal and carbothermal reduction. Plasma-chemical synthesis (vapor-gas phase deposition) is also used to obtain carbide nanopowders. A characteristic is given to each of these methods. Information on the possible mechanism of the processes of carbothermal synthesis is presented.
Keywords
For citations:
Krutskii Yu.L., Gudyma T.S., Krutskaya T.M., Semenov А.О., Utkin A.V. Carbides of transition metals: Properties, application and production. Review. Part 2. Chromium and zirconium carbides. Izvestiya. Ferrous Metallurgy. 2023;66(4):445-458. https://doi.org/10.17073/0368-0797-2023-4-445-458
Introduction
Chromium and zirconium carbides exhibit a range of distinct properties, such as refractoriness, substantial chemical resistance in diverse aggressive environments, elevated hardness, as well as impressive thermal and electrical conductivity. As a result, their utilization within the realms of industry and technology has been steadily increasing. Chromium carbide finds practical application as a constituent in wear-resistant coatings, and it also serves a role in the production of tungsten-free hard alloys and carbide steels. Another avenue of application lies in its function as an inhibiting additive in tungsten carbide hard alloys. The substantial hardness of zirconium carbide renders it suitable as an abrasive for the refinement and polishing of metal products. The primary techniques for synthesizing transition metal carbides encompass carbothermal, metal-thermal, and elemental source-based synthesis methods.
The objective of this study is to conduct an analysis of information pertaining to the properties, applications, and methodologies involved in the synthesis of chromium and zirconium carbides.
Basic properties of chromium and zirconium carbides
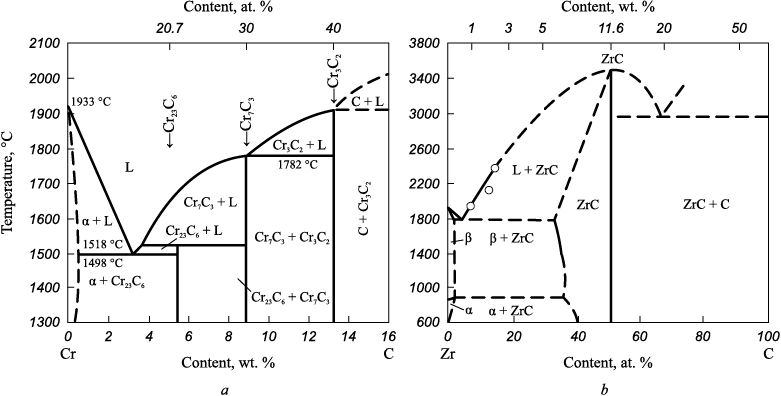
Figure depicts state diagrams for the Cr – С and Zr – С systems [1]. Within the Cr – C system, three distinct carbides (Cr23C6 , Cr7C3 and Cr3C2) with fixed compositions are presented. Carbide Cr3C2 exhibits the highest melting temperature, approximately 1900 °C. When the carbon content within the considered system surpasses 40 at. %, carbon coexists with Cr3C2 carbide. For the production of powdered higher chromium carbides devoid of free carbon impurities, the synthesis temperature should theoretically remain below 1900 °C, aligning with the composition required for obtaining the Cr3C2 reaction product. In practice, even minor inconsistencies in the mixture (such as those caused by delamination during extended storage or vibration) can lead to the formation of a liquid phase within the system at temperatures as low as around 1500 °C. Hence, it is prudent to conduct the synthesis at temperatures not exceeding this threshold.
State diagrams of the systems Cr – C (a) and Zr – C (b) |
In the Zr – C system, a solitary compound, zirconium carbide, prevails, characterized by a wide range of homogeneity (approximately from 35 to 50 at. % C). Zirconium carbide ZrC boasts a melting point of about 3530 °C. A significant reduction in the melting point of zirconium carbide occurs as the carbon content diminishes (1800 °C at approximately 35 at. % C). Likewise, the microhardness experiences a notable decrease (with a C/Zr atomic ratio of 1.0, the value is 3100 kg/mm2, and at C/Zr = 0.6, it is 1900 kg/mm2) [2]. When the carbon content surpasses 50 at. %, carbon coexists with zirconium carbide. Consequently, to produce powdered zirconium carbide devoid of free carbon, while maintaining its high melting point and microhardness in the resulting ceramic, the synthesis temperature should not surpass 3530 °C. Moreover, the mixture’s composition should align with the target reaction product, ZrC composition.
Information pertaining to certain properties of these compounds, drawn from [3], has been consolidated in Table. Both chromium and zirconium carbides exhibit robust thermodynamic stability, substantiated by their elevated heat of formation from basic elements and their isobaric-isothermal potentials. These carbides demonstrate commendably high coefficients of thermal conductivity and low resistivity, mirroring their metal-like refractory nature [4].
Basic thermodynamic, physical and mechanical properties of chromium and zirconium carbides
| |||||||||||||||||||||||||||||
Examination of the chemical attributes of refractory compounds facilitates the formulation of guidelines for their application within diverse aggressive environments. The carbides in question exhibit stability when subjected to base solutions and numerous mineral acids. Additionally, they display resilience against the effects of elevated temperatures and atmospheric oxygen [5].
Areas of application of chromium and zirconium carbides
Application of chromium carbide
Chromium carbide is primarily employed as a constituent within surfacing mixtures, serving to create coatings that safeguard against intensive abrasive wear, including elevated temperatures of up to 800 °C within oxidizing environments. These coatings are applied through methods such as cladding or sputtering. The wear-resistant layer produced through cladding comprises chromium carbide set within a matrix of chromium, nickel [6], or chromium-nickel alloys [7 – 9]. Domestic industry has successfully mastered the production of the powder surfacing tape PL-AN111, which incorporates chromium carbide into the core powder composition. This tape finds utility in restoring surfaces within the contact and intermediate zones of blast furnace charging device cones that operate under forced conditions [10]. Another area of application lies in the creation of cermets, specifically tungsten-free hard alloys of KKhN grades (chromium carbide–nickel) [11]. Furthermore, promising prospects are observed for relatively cost-effective chromium carbide hard alloys and carbide steels with binders composed of iron and chromium, such as the Kh17N2 and Kh13M2 grades [12]. Notably, within the WС – Co hard alloy system, chromium carbide, along with vanadium carbide, serves as a grain growth inhibitor. Information regarding the properties and applications of hard alloys is expounded upon in [13]. The integration of inhibiting additives, such as these carbides, into hard alloys is commonly achieved through electrospark plasma sintering [14 – 16]. However, alternative techniques including microwave heating [17], hot isostatic pressing [18], and non-pressure sintering [19] can also be employed for this purpose. Both chromium and vanadium carbides similarly function as grain growth inhibitors within analogues of WС – Co cermets, exemplified by composite WC – 10 wt. % Si3N4 [20], as well as within cermets founded upon titanium carbonitride Ti(C, N) [21]. Porous materials crafted from chromium carbide–nickel alloys exhibit marked corrosion resistance in both acidic and alkaline solutions [22]. Furthermore, chromium carbide finds application as a catalyst in the oxidation of ammonia and carbon monoxide [23].
Application of zirconium carbide
Zirconium carbide serves as an abrasive material in pastes used for the finishing and lapping of both ferrous and non-ferrous metal components [24]. Beyond its notable hardness, an added benefit lies in its relatively elevated thermal conductivity, which mitigates the risk of burns. Carbon–carbon composites (С – С) display promise as materials suited for high-temperature applications. In this context, particularly when exposed to high-speed airflow, effective protection of the (С – С) composite against high-temperature ablation is achieved through a coating of zirconium carbide. This is due to its resistant to thermal shocks [25; 26].
Chromium and zirconium carbide manufacturing methods
The properties of refractory compounds are contingent on factors like impurity content, dispersity, and stoichiometric balance. Zirconium carbide’s microhardness [2] varies with its composition. Therefore, addressing specific challenges associated with refractory compounds necessitates the meticulous selection of appropriate preparation methods and the determination of acceptable impurity levels within initial constituents. This reality has led to the proliferation of diverse synthesis techniques, which are classified in [27].
The most prevalent methods for carbide synthesis encompass:
– synthesis from elemental constituents
| хМе + уС → МехСу ; | (1) |
– metallothermal (often magnesiothermal) reduction of oxides in the presence of carbon
| МеО + Mg + C → MeC + MgO; | (2) |
– carbothermal reduction of oxides
| МеО + С → МеС + СО. | (3) |
Synthesis reactions of refractory compounds (carbides) from elemental constituents are invariably exothermal. If the heat release reaches a threshold of 2400 kJ/kg of the mixture, the reaction initiates spontaneously. Should the heat release be inadequate, measures such as blend preheating or the utilization of mechanical activation of its components become necessary. Conversely, excessive heat release calls for the incorporation of inert additives into the mixture. These procedures are referred to as SHS processes (self-propagating high-temperature synthesis). Under optimal conditions, nearly complete conversion of the initial substances into the final products transpires, typically yielding an unreacted substance content of around 1 % by mass. Since the synthesis process is devoid of contamination, the product’s purity in terms of impurities is roughly equivalent to that of the reagents [28]. Nevertheless, such processes are encumbered by the high cost of elemental powders.
During the metallothermal synthesis of carbides, it becomes imperative to subject the reaction products to treatment (typically with an acid) in order to eliminate compounds, notably oxides, stemming from the reducing metal – commonly magnesium. Owing to magnesium’s comparably low boiling point (1090 °C) [29] and the substantial heat liberation inherent in magnesiothermal processes, the emission of hot blend and reaction byproducts is plausible. To preempt such scenarios, these processes are conducted within sealed reactors operating under elevated argon pressure. A notable attribute of magnesiothermal and calciumthermal reduction processes is the emergence of refractory compound particles enshrouded by layers of magnesium or calcium oxides characterized by elevated melting points [30]. Consequently, the resultant products of the reaction exhibit high dispersion. Moreover, it’s essential to factor in the high cost and toxic nature of powdered magnesium [31].
It is widely considered [4; 32] that the carbothermal synthesis of carbides stands as the most promising approach for the large-scale production of these compounds. In the carbothermal method for obtaining transition metal carbides, the reagents utilized are typically non-toxic. The processes of carbide formation occur within the solid phase. Given the endothermic nature of carbide formation, these processes are conducted at elevated temperatures. To optimize synthesis parameters, the carbon monoxide (CO) pressure is reduced by performing the process in an inert gas environment or under vacuum conditions. Carbothermal reduction can also be accomplished via the sol–gel method. A distinctive trait of this approach lies in its relatively lower synthesis temperatures, which arise from the close contact between reagents within ultra-dispersed blends [3]. The resultant products are in a nanodispersed state. Nonetheless, drawbacks of the sol–gel method encompass the use of toxic reagents in several syntheses, the intricacy (including duration and multi-stage nature) of batch preparation processes, and the occurrence of incomplete reaction conversion in certain cases.
Higher chromium carbide manufacturing method
Synthesis through chromium and carbon
For chromium carbide Cr3C2 , the heat of formation aligns closely with its enthalpy at relatively low temperatures (approximately 1140 K) [3]. As a result, initiating the SHS process for a mixture of chromium and carbon at ambient temperature is impractical. Instead, this synthesis can be realized through preheating the mixture. In the case at hand, mixture’s mechanical activation typically preceded the SHS process.
Mechanical activation alone doesn’t inherently lead to the synthesis of chromium carbides. Research conducted in [33] demonstrated that fullerenes exhibit greater reactivity compared to graphite. While mechanical activation aids in reducing synthesis parameters (for instance, the process temperature for Cr3C2 carbide synthesis can be lowered by around 200 °C) [34], incomplete carbide formation reactions were noted in [35; 36]. Nevertheless, employing high-energy mechanical activation enables the production of compact Cr3C2 carbide products [37]. It’s worth noting that mechanical activation is a time-consuming (often spanning several hours) and energy-intensive procedure.
An alternative approach involves elevating the thermality of the process. As explored in [38], the synthesis of chromium carbides can occur when a portion of carbon black is replaced with polytetrafluoroethylene (–CF2 = CF2–)n . At temperatures ranging from 900 to 1000 K, this compound decomposes, yielding graphite and fluorine. The latter substance interacts with chromium, forming chromium fluorides. This reaction releases substantial heat, consequently triggering an SHS process for Cr7C3 carbides or Cr3C2 carbides with particle sizes measuring 0.5 – 2.0 µm. It is imperative to purify the reaction products from chromium fluoride CrF2 . Given the involvement of fluorine, the reactor necessitates sealing.
Metallothermal reduction
The procedure for producing cast chromium carbide through the SHS process is documented [39]. The reagents employed included chromium oxides (Cr2O3 and CrO3 ), aluminum and graphite powders. This synthesis took place within an argon environment under pressures ranging from 4 to 20 MPa. The material had to be comminuted to attain the necessary powder. The reaction products contained up to 3 wt. % of aluminum, necessitating subsequent removal through acid treatment. These factors collectively add complexity to the process. Notably, CrO3 oxide is highly toxic [40]. An alternate approach, the aluminothermal process, was described in [41] for synthesizing chromium carbide. While initially, chromium was reduced from aluminum oxide, the reaction mixture was subsequently maintained in an argon environment at 800 °C for two hours. The resulting product (effectively Cr3C2 – Al2O3 composite) consisted of particles sized between 300 and 400 μm.
Additionally, [42] explored the synthesis of chromium carbide through the magnesiothermal method:
| 3Cr2O3 + 9Mg + 4C = 2Cr3C2 + 9MgO. | (4) |
Due to the process’s inherent high adiabatic temperature (1950 °C), the introduction of an inert additive (Cr3C2 ) into the mixture was undertaken. This procedure transpired within an argon environment. The average size of the produced chromium carbide particles measures 2 μm. The magnesiothermal synthesis of chromium carbide is elucidated in [43]. The concoction of chromium oxide, magnesium, and acetone (as the carbon source) underwent thermal treatment within an autoclave at a temperature of 700 °C. The reagents were combined in a stoichiometric proportion to enable the reaction:
| 3Cr2O3 + 10Mg + 2C3H6O = 2Cr3C2 + 10MgO + C + CO + 6H2 . | (5) |
Following a 15 h isothermal holding, chromium carbide with particle sizes ranging from 35 to 50 nm, enveloped by carbon layers measuring 3 – 4 nm in thickness, was successfully obtained.
Carbothermal reduction
The carbothermal reduction of chromium oxide is conducted through the comprehensive reaction:
| 3Сr2О3 + 13C = 2Cr3C2 + 9СО. | (6) |
This process involves the reduction of thermodynamically robust chromium oxide through the utilization of a relatively feeble reducing agent, carbon monoxide (CO). In this context, the role of carbon is primarily focused on regenerating the generated carbon dioxide CO2 [44]. From a thermodynamic standpoint, this process might seem implausible. Conclusions drawn from [45] suggest that the formation of chromium carbides is likely to transpire during the interaction between chromium oxide and solid carbon. In [46], the process of reducing chromium oxide using various agents (lamp carbon black, petroleum coke, thermal anthracite, semi-coke, and graphite) was investigated. The content of the reducing agent in the mixture corresponded to the stoichiometric ratio for reaction (3). The study revealed that the reduction start temperature displays weak dependency on the type of carbon material, spanning within the range of 1050 – 1100 °С. The gaseous phase predominantly consists of carbon monoxide, CO. Among the various environments, the most robust carbide formation occurs in hydrogen, followed by helium, with vacuum hosting the slowest process. Notably, the reaction products comprise a blend of Cr3C2 and Cr7C3 carbides. Authors in [46] propose carbon monoxide, CO, as the reducing agent for chromium oxide (contrary to thermodynamics). Meanwhile, [47] presents results from an exploration of chromium oxide interaction with diverse carbon materials (sucrose, carbon fiber material (CFM), and carbon black). The carbon fiber material consists of carbonization products from hydrated cellulose fibers. The mixture’s reducing agent content corresponded to the stoichiometric value for reaction (6). Based on X-ray diffraction data, sucrose initiates Cr3C2 phase formation at 1200 °C, while CFM and carbon black begin at 1350 °C and 1400 °C, respectively. The lowered temperature for carbide formation with organic substances (sucrose and hydrocarbons) can be attributed to the creation of highly dispersed carbon components resulting from their thermal degradation within the oxide mixture.
Further insights into this process, focusing on gas phase control (continuous monitoring of CO and CO2 oxides) are presented in [48; 49]. The charging material was heated within an inert gas atmosphere (helium or argon). At approximately 900 °C, a preference for carbon dioxide CO2 emission over carbon monoxide CO emission was observed. This behavior is linked to carbon’s interaction with adsorbed acidic species on its surface. Notably, the carbide phase predominantly forms on the surface of chromium oxide particles [4]. Following the establishment of an outer Cr3C2 carbide layer, interaction with chromium oxide commences, resulting in the formation of chromium carbide with a Cr7C3 composition. In cases where the initial reagent mixture contains insufficient carbon, the resulting product would exhibit a mixed-phase composition. The maximum liberation of carbon monoxide CO occurs at approximately 1200 °C (with a low content of carbon dioxide CO2 at this temperature). If the reduction process followed the mechanism postulated in [44], the of CO and CO2 oxides in the gas phase during chromium oxide reduction would be comparable.
The vapor pressure of chromium oxide at 1700 K (approximately 1430 °C) is approximately 7·10\(^ - \)5 mm Hg (equivalent to about 9·10\(^ - \)3 Pa), which is close to that of carbothermal synthesis. The vapor consists of chromium atoms, oxygen, and molecules of CrO, CrO2 , O2 [50]. In contrast, the vapor pressure over carbon at the same temperature is significantly lower, measuring 9.13·10\(^ - \)14 atm (approximately 9·10\(^ - \)9 Pa) [8]. It is known that the evaporation of chromium oxide is notably enhanced in the presence of carbon [49].
The process of synthesizing chromium carbide partially involves the transfer of vaporous chromium and its oxides to the surface of the carbon reducing agent. This phenomenon is supported by the findings in references [51; 52].
Another perspective suggests that the carbothermal reduction process occurs through the direct interaction between solid oxide and carbon. According to the Cr – C system’s phase diagram, a liquid phase may emerge at temperatures exceeding 1498 °C, facilitating close contact between the oxide and carbon and expediting the reduction process [51].
To summarize the aforementioned points, it can be inferred that the carbothermal reduction process of chromium oxide is quite intricate and may follow several mechanisms. Determining which one predominates is challenging.
In order to obtain reaction product consisting solely of carbide, briquettes composed of a calculated blend with an additional 5 % dextrin solution were heated in a resistance furnace to 1500 °C for 30 – 40 min and maintained at this temperature for 1.5 – 2.0 h in a hydrogen environment. The resulting carbide contained minimal impurities (wt. %: 87 of total Cr; 13.48 total C; 13.34 C bonded with a theoretical content of 86.67 Cr and 13.33 C) and exhibited an average particle size of 6.94 μm [53]. Reference [54] explored a process for producing chromium carbide using nano-sized powders of chromium oxide (average particle size less than 60 nm) and carbon black with a 14 % excess (average particle size less than 50 nm), nearly following a stoichiometric ratio to carry out reaction (3). The reduction initially yielded the lowest oxide, CrO. A single-phase product containing only Cr3C2 carbide was obtained at 1200 °C and held at that temperature for one hour. This suggests that under such conditions, the carbide formation process was completed. However, thermogravimetric analysis results revealed that even at 1200 °C, the weight loss (15.7 wt. %) was much lower than the value calculated based on the assumption of complete reaction (3) (41.2 wt. %). The average particle size of chromium carbide was approximately 50 nm. With an increase in synthesis time, the particles grew in size and aggregated.
In [55], a mixture of powdered chromium oxide Cr2O3 , along with graphite or synthetic pitch, was subjected to heating in an argon gas environment, with the addition of 5 vol. % water, at varying temperatures for two hours. Experimental findings indicated that when synthetic pitch was employed as the reducing agent, a single-phase product (Cr3C2) formed at 1100 °C, whereas when graphite was used, this phase appeared at 1300 °C. This implies that synthetic pitch functions as a more active reducing agent compared to graphite. However, it is worth noting that the manufacturing process is time-consuming and labor-intensive. Details concerning particle dispersion were not provided. Reference [56] employed ammonium dichromate as a chromium source and carbon black as the carbon source. The mixture was heated in a vacuum environment at 1100 °C for a duration of 30 min, resulting in the formation of a single-phase product (Cr3C2 carbide). The produced powders were primarily composed of spherical particles with an average size of 27.2 nm. X-ray photoelectron spectroscopy revealed the presence of not only chromium and carbon but also oxygen. Therefore, under these specific conditions, the carbide formation process remains incomplete. The authors [56] suggest that the creation of chromium carbide progresses through the development of chromium carbide proceeds through the formation of an intermediate carbide phase
Cr3C2 – x(0 ≤ x ≤0.5) : Cr2O3 → Cr3C2 – x → Cr3C2 .
The synthesis of chromium carbide utilizing a novel type of carbon material known as nanofiber carbon (NFC), obtained through catalytic decomposition of light hydrocarbons, is outlined in references [57; 58]. This material is notably pure, with impurities mainly comprising catalyst remnants, constituting no more than 1 wt. %. It is distinguished by its substantial specific surface area (approximately 150 m2/g) [59]. Experiments were conducted in an argon environment. Under optimized conditions, the resulting material is characterized by a single phase (chromium carbide Cr3C2). Powder particles predominantly manifest aggregation. The average size of particles and aggregates measures 7.8 µm, displaying a wide array of size distributions. The specific surface area of the samples is 2.2 m2/g. Oxidation of chromium carbide commences at 640 °C and nears completion at 1000 °C. The ideal synthesis parameters were identified as a molar ratio of Cr2O3:C = 3:13 (stoichiometric ratio for achieving Cr3C2 compound), and process temperature of 1300 – 1400 °С. Mention exists of the synthesis of chromium carbide using this method from ultrafine blends in references [60; 61]. Reference [60] formed a precursor (chromium tartrate) through a combination of chromium oxide CrO3 solutions and tartaric acid, followed by drying. Similarly, in [61], a precursor was obtained from ammonium bichromate (NH4)2Cr2O7 and glucose. Heat treatment of the blends was performed in argon at 1100 °C [60] or in a vacuum environment (10\(^ - \)2 Pa) [61]. Particle sizes measured 1 – 2 µm [60] or approximately 30 nm [61].
Methane [62], along with its blends containing hydrogen [63] or argon [64; 65], can serve as effective reducing agents. The temperatures required for carbide formation are lower compared to those when solid carbon materials are used. While it is true that thermodynamically speaking, the use of hydrocarbons can lower the initiation temperature of reduction, this approach adds complexity to the process and raises concerns about its fire and explosion risks.
Vapor deposition
In [66], the production of ultrafine chromium carbonitride powder of Cr3(C0.8N0.2)2 , through plasma-chemical synthesis was explored. This process involved reducing chromium oxide using a nitrogen-hydrogen plasma flow containing propane–butane. However, the exhaust gases generated during this process contain hazardous hydrogen cyanide. The resulting powder had an average particle size of 35 nm, with the primary substance content ranging from 90.23 to 94.60 wt. %. It is worth noting that when stored in air, the chromium carbonitride powders exhibited significant oxygen and moisture adsorption. In comparison to coarser-grained chromium carbide powders [67], the thermal oxidative stability of this compound is relatively lower: oxidation initiates at approximately 280 °C and is nearly completed at 580 °C. A similar process was examined in reference [68]. Experimental results indicated that the interaction between chromium oxide and hydrocarbons within a plasma flow did not yield higher single-phase carbides. Another study in [69] described attempts to produce chromium carbonitride in a nitrogen plasma flow through the interaction of chromium metal powder and natural gas containing 94 vol. % methane. The resulting synthesis products contained 91.8 – 93.5 wt. % of the target compound, with particle sizes ranging from 150 to 600 nm.
In many of the referenced works ([33 – 35; 38; 39; 41 – 43; 45 – 47; 49; 51; 52; 54 – 56; 60; 62 – 65; 68] which account for approximately 83 % of the total cited references, information about the content of impurities in the final product was not provided.
Zirconium carbide manufacturing method
Synthesis through zirconium and carbon
In [70], zirconium and acetylene carbon black powders employed as initial materials. The blend, with a stoichiometric composition, underwent “dry” stirring for 2 – 3 h. Subsequently, it was compacted into briquettes to expedite diffusion processes. The synthesis occurred under a pressure of 1.2·10\(^ - \)4 mm Hg (0.2 Pa). At a temperature of 1800 °C and a duration of one hour, zirconium carbide (ZrC) was successfully produced. The presence of impurities in the final product amounted to 0.6 wt. %. However, no information regarding particle dispersion was provided.
Metallothermal reduction
In [71 – 73], magnesiothermal synthesis was conducted according to the reaction
| ZrO2 + 2Mg + C = ZrC + 2MgO. | (7) |
To mitigate the process’s thermal intensity, an inert additive, such as sodium fluoride, was utilized [71], or blends were formulated with an excess of magnesium beyond stoichiometric proportions [72]. In [73], zirconium carbide was obtained after subjecting the materials to 30 h of mechanical activation. An alternative means of initiating magnesiothermal reduction involves partially oxidizing magnesium through its interaction with water [74]. This procedure leads to a high-pressure environment due to the evolution of hydrogen (pressure reaching 49.15 MPa). The resultant zirconium carbide nanoparticles were approximately 500 nm in size.
In the examined process, sodium [75] can also serve as a reducing metal. The process in question involved the reagents zirconium chloride ZrCl4 , sodium and toluene. By heating the solid residue produced after the excess toluene had evaporated, zirconium carbide was synthesized at a temperature of 700 °C over the course of one hour in an argon environment. It’s important to note that sodium is highly susceptible to oxidation in air [40], which posed challenges during the blend preparation stage.
Carbothermal reduction
The comprehensive reaction equation is as follows
| ZrO2 + 3C = ZrC + 2CO. | (8) |
Researchers [76] posit that the reduction of ZrO2 oxide, which is thermodynamically robust, takes place through carbon rather than the relatively thermodynamically weaker reducing agent (CO). The interaction likely involves the transfer of oxide vapors (ZrO2 , ZrO [50]) to the oxide’s surface, followed by chemical interaction and desorption of the gaseous product (CO) resulting from the reaction. Conversely, researchers [44] lean towards the notion that CO drives the reduction of zirconium oxide, with carbon’s role limited to regeneration. However, this viewpoint lacks substantial experimental support. In [77], active carbon, carbon black, and graphite powder served as carbon materials. These reagents were proportioned stoichiometrically to facilitate reaction (8). Monitoring of the reduction process was based on the quantity of released CO oxide. At temperatures of 1800 and 2000 °C, the conversion degree approached 100 %, and the oxygen content in the reaction products remained below 1 wt. % (except for instances involving thermal treatment with graphite). Carbothermal regeneration processes of titanium and zirconium oxides share certain similarities, given that zirconium carbide forms from oxycarbide ZrOxCy . The particle size of the obtained material predominantly fell within the range of 2.4 – 7.5 μm, accounting for 90 wt. %. Reference [48] also utilized active carbon, carbon black, and graphite powder as carbon materials. The blend was prepared in stoichiometric proportion for reaction (8) and subsequently heated in an inert gas flow (helium). The oxycarbide phase ZrOxCy emerged at 1450 °C, with the ZrO2 phase already absent in samples synthesized at 2000 °C. Oxygen content in these samples remained around 0.6 wt. %. Notably, at synthesis temperatures exceeding 1450 °C, the gas phase primarily comprised CO oxide. This serves as unequivocal evidence against the feasibility of reducing zirconium oxide ZrO2 using carbon monoxide CO, as such a reaction would yield a significant quantity of oxide CO2 in the gas phase. However, particle size information is not provided. From a thermodynamic standpoint, reactions [78] are infeasible
| ZrO2 + CO = ZrC + 1.5O2 ; | (9) |
| ZrO2 + 4CO = ZrC + 3CO2 . | (10) |
In [78], samples composed of compressed zirconium oxide ZrO2 and graphite were subjected to heating within a helium environment. The formation of the ZrC phase took place at 1800 °C. The primary mechanism behind zirconium carbide formation predominantly involves carbon diffusion into zirconium oxide ZrO2 , as opposed to the vapor transfer of ZrO2 and ZrO oxides to the carbon surface, followed by chemical interaction).
An examination of the aforementioned published data yields the following insights. Challenging is the alignment with the viewpoint of the authors in [44] concerning the reduction of zirconium dioxide with carbon monoxide. More recent experimental findings [79] suggest that the reduction process more likely transpires through the transfer of zirconium oxide vapors to the carbon material’s surface, accompanied by a chemical reaction and subsequent removal of the resulting gaseous product (CO). While the possibility of solid-phase interaction involving carbon diffusion into zirconium dioxide is not excluded, reduction leads to the formation of zirconium oxycarbide ZrOxCy , with its oxygen content diminishing over time.
The process of generating zirconium carbide via the interaction between zirconium dioxide and carbon was outlined in [79 – 81].
A thermodynamic analysis of the process for obtaining zirconium carbide and its synthesis through heating a compressed mixture with an argon-hydrogen plasma was conducted in [79]. When employing a reactant ratio (ZrO2 + C) in accordance with the stoichiometry of Eq. (5), the degree of transformation of zirconium dioxide into carbide approached unity within the temperature range of 1900 – 3800 K. In experiments involving a stoichiometric reactant ratio and a thermal treatment time of three minutes, the resulting product was single-phase (ZrC) with a carbon content of 4.14 wt. % and an oxygen content of 0.35 wt. %. However, information regarding the dispersity of zirconium carbide was not provided. Zirconium carbide was synthesized in [80] using zirconium dioxide and carbon black. The mixture of calcined components was ball-milled for eight hours and subsequently heated in a hydrogen environment. Optimal outcomes were achieved at a temperature of 2200 °C and a holding time of 60 min. The content of bonded carbon amounted to 11.30 wt. %, while the calculated content was 11.65 wt. %. Unfortunately, no information concerning powder dispersity was provided. In [81], the reagents comprised zirconium dioxide and graphite powders, with the blend’s composition aligning with the stoichiometry of Eq. (8). Consequently, the reduction of zirconium dioxide to carbide does not involve carbon oxide (CO). The blend underwent high-energy grinding in a planetary mill for 20 h, followed by heat treatment at temperatures ranging from 1300 to 1600 °C for two hours under vacuum conditions. Full transformation of the reactants was achieved at 1400 °C. This indicates that the utilization of high-energy grinding enables a reduction in the carbide formation temperature by 400 °C. No reports of reagent contamination with grinding media and lining materials were made. The resulting powdered material consisted of agglomerates measuring approximately 7 μm in size, comprised of particles around 200 nm in size.
The synthesis of zirconium carbide utilizing nanofibrous carbon, characterized by its low impurity content (around 1 wt. %) and substantial specific surface area (approximately 150 m2/g) [59], was explored in references [82; 83]. The blend was formulated in accordance with the stoichiometry of Eq. (5). It was observed that the use of such material led to a reduction in the process temperature by approximately 200 °C, ultimately yielding a highly dispersed single-phase product (ZrC) with an average particle size of approximately 15 μm. The impurity content remained relatively low, approximately 2 wt. %.
The series of publications provides comprehensive insights into the zirconium carbide production process via the carbothermal method from ultrafine blends. Zirconium butoxide Zr(OC4H9)4 [84 – 86], zirconium n-propoxide Zr(OC3H7)4 [87 – 89], zirconium tetrachloride ZrCl4 [90 – 92], zirconium nitrate Zr(NO3)4∙5H2O [93], and zirconium oxychloride ZrOCl2∙8H2O were utilized as zirconia sources [94 – 96]. The carbon sources encompassed propanol C3H7OH [95], butanol C4H9OH [84; 85], furfuryl alcohol С5Н5ООН [92], acetylacetone СН3–СО–СН2–СО–СН3 [89; 96], sucrose [87; 95], phenolic resin [86; 88; 91], salicylic acid С6Н4–ОН–СООН [89], triethylamine C6H15N [89], divinylbenzene C6H4(C2H5)2 [90], glucose [94; 96], chitosan [93], 1,4-butanediol С4Н10О2 [96]. The reagents containing zirconium and carbon were stirred for 1 – 3 h. Subsequently, the solvent was removed either through evaporation or by subjecting the mixture to vacuum conditions. The resulting dry residue (ultrafine mixture) underwent heat treatment within an inert gaseous medium or under vacuum at conditions at temperatures ranging from 1250 to 1600 °C.
It is intriguing to note that in nearly all the aforementioned publications, the authors carried out calculations based on reaction (8) during blend preparation. Consequently, they hold the belief that the reduction of zirconium carbide from the oxide takes place through solid carbon, rather than involving carbon monoxide СО.
Vapor deposition
Zirconium carbonitride ZrC0.90N0.06 was synthesized within a nitrogen-hydrogen plasma flow through the reduction of zirconium oxide ZrO2 with propane-butane [66]. The content of impurities (ZrO2 and free carbon, Cfree ) within the resulting reaction products ranged from 16.88 to 19.95 wt. %. One potential explanation for such a high impurity content lies in the considerable thermodynamic stability exhibited by zirconium oxide [11]. The average particle size of the synthesized material measured around 50 nm. It’s essential to highlight that the exhaust gases produced during this process contain toxic hydrogen cyanide. Additionally, observations indicated that the zirconium carbonitride powders, when exposed to air, exhibited a pronounced propensity for oxygen and moisture adsorption.
Synthesis through salt melt electrolysis
In [97], zirconium carbide powder with particle size ranging from 60 to 100 nm was successfully produced through electrolysis in a calcium chloride melt at a temperature of 1123 K (850 °C). A compressed mixture of ZrO2/C was utilized as the anode, and the process ran for a duration of seven hours.
In several of the cited publications ([73 – 75; 78; 81; 84; 85; 89 – 92; 95 – 97], amounting to approximately 65 % of the total references), no information regarding the content of impurities in the final product was provided.
Conclusions
This text discusses refractory oxygen-free metal-like compounds, specifically chromium and zirconium carbides, highlighting their properties and applications. Chromium and zirconium carbides are characterized by high thermal and electrical conductivity, notable hardness, and chemical inertness. They find application across various engineering fields, such as abrasives, wear-resistant ceramics, and components in surfacing materials. Chromium carbide is utilized in tungsten-free hard alloys and carbide steels. Zirconium carbide shows promise as an inhibitory additive in hard alloy production and can function as a catalyst in organic synthesis. The methods for preparing these compounds are detailed and analyzed, outlining their distinctive features.
The majority of research on the synthesis of chromium and zirconium carbides focuses on their production from simple substances through carbothermal and metallothermal processes. Limited information exists on the preparation of these compounds via vapor-gas deposition. A noteworthy observation is that many cited publications lack details about the purity of the reaction products, often relying solely on X-ray phase analysis to gauge the completeness of the carbide formation processes. A plausible mechanism for the formation of these compounds involves the transfer of oxide vapors to the surface of carbon material particles, followed by subsequent chemical interaction. Additionally, it’s conceivable that carbides may form through direct contact between solid reagents.
References
1. State Diagrams of Binary Metal Systems. Reference book. Lyakishev N.P. еd. Moscow: Mashinostroenie; 1996;1:992. (In Russ.).
2. Vinitskii I.M. Dependence of properties of monocarbides of IV-V groups transition metals on carbon content. Poroshkovaya metallurgiya. 1972;(6):76–82. (In Russ.).
3. Properties, Production and Application of Refractory Compounds. Kosolapova T.Ya. еd. Moscow: Metallurgiya;1986:928. (In Russ.).
4. Kosolapova T.Ya. Carbides. Moscow: Metallurgiya; 1968: 300. (In Russ.).
5. Kosolapova T.Ya. Chemical properties of refractory compounds. Zhurnal VHO im. D.I. Mendeleeva. 1979;24(3):244–249. (In Russ.).
6. Wang L., Zhou J., Yu Y., Guo C., Chen J. Effect of powders refinement on the tribological behavior of Ni-based composite coatings by laser cladding. Applied Surface Science. 2012;258(17):6697–6704. http://doi.org/10.1016/j.apsusc.2012.03.141
7. Matthews S., James B., Hyland M. The role of microstructure in the high temperature oxidation mechanism of Cr3C2–NiCr composite coatings. Corrosion Science. 2009;51(5): 1172–1180. http://doi.org/10.1016/j.corsci.2009.02.027
8. Chatha S.S., Sidhu H.S., Sidhu B.S. High temperature hot corrosion behavior of NiCr and Cr3C2–NiCr coatings on T91 boiler steel in an aggressive environment at 750 °С. Surface and Coating Technology. 2012;206(19-20):3839–3850. http://doi.org/10.1016/j.surfcoat.2012.01.060
9. Kaur M., Singh H., Prakash S. High-temperature behavior of a high-velocity oxy-fuel sprayed Cr3C2–NiCr coating. Metallurgical and Materials Transactions A. 2012;43:2979–2993. http://doi.org/10.1007/s11661-012-1118-4
10. Yuzvenko Yu.A. Surfacing. Kyiv: Naukova dumka; 1976:71. (In Russ.).
11. Klimenko V.N., Maslyuk V.A. Corrosion-resistant metal-ceramic alloys based on chromium carbide. Tekhnologiya i organizatsiya proizvodstva. 1983; 3: 82–85. (In Russ.).
12. Maslyuk V.A., Yakovenko R.V., Potazhevskaya O.A., Bondar A.A. Powdered hard alloys and chromium carbides based on the Cr–Fe–C system. Poroshkovaya metallurgiya. 2013;(1/2):60–74. (In Russ.).
13. Kurlov A.S., Gusev A.I. Physics and Chemistry of Tungsten Carbides. Moscow: Fizmatlit; 2013:272. (In Russ.).
14. Bonache V., Salvador M.D., Rocha V.G., Borrell A. Microstructural control of ultrafine and nanocrystalline WC–12Co–VC/Cr3C2 mixture by spark plasma sintering. Ceramics International. 2011;37(3):1139–1142. http://doi.org/10.1016/j.ceramint.2010.11.026
15. Chen H., Yang Q., Wang J., Yang H., Chen L., Ruan J., Huang Q. Effects of VC/Cr3C2 on WC grain morphologies and mechanical properties of WC–6 wt. % Co cemented carbides. Journal of Alloys and Compounds. 2017;714:245–250. https://doi.org/10.1016/j.jallcom.2017.04.187
16. Sun L., Tian’en Y., Jia C., Hiong J. VC, Cr3C2 doped ultrafine WC–Co cemented carbides prepared by spark plasma sintering. International Journal of Refractory Metals and Hard Materials. 2011;29(2):147–152. http://doi.org/10.1016/j.ijrmhm.2010.09.004
17. Zhao Z. Microwave-assisted synthesis of vanadium and chromium carbides nanocomposite and its effect on properties of WC–8Co cemented carbides. Scripta Materialia. 2016;120:103–106. http://doi.org/10.1016/j.scriptamat.2016.04.024
18. Bonache V., Salvador M.D., Fernández A., Borrell A. Fabrication of full density near-nanostructured cemented carbides by combination of VC/Cr3C2 addition and consolidation by SPS and HIP technologies. International Journal of Refractory Metals and Hard Materials. 2011;29(3):202–208. http://doi.org/10.1016/j.ceramint.2010.11.026
19. Espinoza-Fernández L., Borrell A., Salvador M.D., Gutierrez-Gonzalez C.F. Sliding wear behavior of WC–Co–Cr3C2–VC composites fabricated by conventional and non-conventional techniques. Wear. 2013;307(1-2):60–67. http://doi.org/10.1016/J.WEAR.2013.08.003
20. Li Y., Zheng D., Li X., Qu S., Yang C. Cr3C2 and VC doped WC–Si3N4 composites prepared by spark plasma sintering. International Journal of Refractory Metals and Hard Materials. 2013;41:540–546. http://doi.org/10.1016/j.ijrmhm.2013.07.004
21. Wan W., Xiong J., Guo Z., Dong G., Yi C. Effects of Cr3C2 addition on the corrosion-erosion behavior of Ti(C,N)-based cermets. Tribology International. 2013;39:178–186. http://doi.org/10.1016/j.triboint.2013.03.019
22. Apininskaya L.M., Klimenko V.N., Maslyuk V.A., Radomyselskii I.D. Porous products made of chromium carbide alloys. Poroshkovaya metallurgiya. 1971;(2):33–36. (In Russ.).
23. Kharlamov A.I., Kirillov N.V. Catalytic properties of powders of refractory compounds of transition elements. Carbides and nitrides. Poroshkovaya metallurgiya. 1983;(2): 55–67. (In Russ.).
24. Adamovskii A.A. Carbides of transition metals in abrasive processing. Poroshkovaya metallurgiya. 2007;(11/12): 96–111. (In Russ.).
25. Sun W., Xiong X., Huang B.-Y., Li G.-D., Zhang H.-B., Chen Z.-K., Zheng X.-L. ZrC ablation protective coating for carbon/carbon composites. Carbon. 2009;47(14):
26. –3371. http://doi.org/10.1016/j.carbon.2009.07.047
27. Wang S.-L., Li K.-Z., Li H.-Y., Zhang Y.-L. Microstructure and ablation resistance of ZrC nanostructured coating for carbon/carbon composite. Materials Letters. 2013;107: 99–102. http://doi.org/10.1016/j.matlet.2013.05.124
28. Gurin V.N. Methods of synthesis of refractory compounds and prospects of their application for new materials creation. Zhurnal VKhO im. D.I. Mendeleeva. 1979;24(3):212–222. (In Russ.).
29. Merzhanov A.G., Borovinskaya I.P. Self-propagating high-temperature synthesis in the chemistry and technology of refractory compounds. Zhurnal VKhO im. D.I. Mendeleeva. 1979;24(3):223–227. (In Russ.).
30. Properties of Elements. Part 1. Physical Properties. Samsonova G.V. еd. Moscow: Metallurgiya; 1976:600. (In Russ.).
31. Physicochemical Properties of Oxides. Samsonova G.V. ed. Moscow: Metallurgiya; 1978: 472. (In Russ.).
32. Samsonov G.V., Perminov V.P. Magnesiothermy. Moscow: Metallurgiya; 1971:176. (In Russ.).
33. Kieffer R., Benezovsky F. Hard Materials. Moscow: Metallurgiya; 1968:384. (In Russ.).
34. Liu Z.G., Tsuchiya K., Umemoto M. Mechanical milling of fullerene with carbide forming elements. Journal of Materials Science. 2002;37:1229–1235. http://doi.org/10.1023/A:1014383909485
35. Li L., Tang J. Synthesis of Cr7C3 and Cr3C2 by mechanical alloying. Journal of Allows and Compounds. 1994;209(1-2): L1–L3. http://doi.org/10.1016/0925-8388(94)91060-X
36. Gomari S., Shafari S. Microstructural characterization of nanocrystalline chromium carbides synthesized by high energy ball milling. Journal of Allows and Compounds. 2010;490(1-2):26–30. http://doi.org/10.1016/j.jallcom.2009.10.041
37. Pripisnov O.N., Shelekhov E.V., Rupasov S.I., Medvedev A.S. The mechanism of phase formation and features of mechanochemical synthesis of chromium carbides. Izvestiya vuzov. Poroshkovaya metallurgiya i funktsional’nye pokrytiya. 2014;(3):8–15. (In Russ.). http://doi.org/10.17073/1997-308X-2014-3-8-15
38. Sharafi S., Gomari S. Effects of milling and subsequent consolidation treatment on the microstructural properties and hardness of the nanocrystalline chromium carbide powders. International Journal of Refractory Metals and Hard Materials. 2012;30(1):57–63. http://doi.org/10.1016/j.ijrmhm.2011.07.004
39. Manukyan H.V., Nersisyan G.A., Kharatyan S.L. Activated chromium-carbon combustion and chromium carbide synthesis. Khimicheskaya fizika. 2001;20(11):34–37. (In Russ.).
40. Gorshkov V.A., Komratov G.N., Yukhvid V.I. Production of cast higher chromium carbide by self-propagating high-temperature synthesis. Poroshkovaya metallurgiya. 1992;(11):57–60. (In Russ.).
41. Rosin I.V., Tomina L.D. General and Inorganic Chemistry. Modern Course. Moscow: Yurait; 2012:1338. (In Russ.).
42. Cintho O.M., Favilla E.A.P., Capocchi J.D.T. Mechanical-thermal synthesis of chromium carbides. Journal of Allows and Compounds. 2007;439(1-2):189–195. http://doi.org/10.1016/j.jallcom.2006.03.102
43. Ko S-K., Won C.-W., Shon I.-J. Synthesis of Cr3С2 by SHS process. Scripta Materialia. 1997;31(6):889–895. http://doi.org/10.1016/S1359-6462(97)00181-4
44. Mahajan M., Rajpoot S., Randey O.P. In-situ synthesis of chromium carbide (Cr3C2) nanopowders by chemical-reduction route. International Journal of Refractory Metals and Hard Materials. 2015;50:113–119. http://doi.org/10.1016/J.IJRMHM.2014.12.010
45. Vodop’yanov A.G., Kozhevnikov G.N., Baranov S.V. Interaction of refractory metal oxides with carbon. Uspekhi khimii. 1988;LVII(9):1419–1439. (In Russ.).
46. Popov A.A., Ostrik P.N., Gasik M.I. Thermodynamics of reduction and formation of carbide in the Cr–C–O system. Izvestiya. Ferrous Metallurgy. 1986;29(10):1–3. (In Russ.).
47. Golodov S.M., Kolchanov V.A., Tarabrin G.K., Sorin S.B. Study of the interaction of chromium oxide with carbon. Izvestiya. Ferrous Metallurgy. 1984;27(5):6–9. (In Russ.).
48. Vlasova V.M., Kakazei N.G., Minakov V.N., Sergeev V.P., Sinel’nikova V.S., Kharlamov A.I., Khorpyakov O.T. Carbide formation in chromium oxide – carbon-containing component systems. Neorganicheskie materialy. 1988;24(12):1998–2003. (In Russ.).
49. Gruner W., Stolle S., Wetzig K. Formation of COх species during the carbothermal reduction of oxides of Zr, Si, Ti, Cr, W and Mo. International Journal of Refractory Metals and Hard Materials. 2000;18(2-3):137–145. http://doi.org/10.1016/S0263-4368(00)00013-5
50. Berger L.-M., Stolle S., Gruner W., Wetzig K. Investigation of the carbothermal reduction process of chromium oxide by micro and lab-scale methods. International Journal of Refractory Metals and Hard Materials. 2001;19(2):109–121. http://doi.org/10.1016/S0263-4368(01)00003-8
51. Kazenas E.K., Tsvetkov Yu.V. Thermodynamics of Oxide Evaporation. Moscow: LKI; 2008:480. (In Russ.).
52. Vodop’yanov A.G., Serebryakova A.V., Kozhevnikov G.I. On the mechanism of interaction of chromium oxide with carbon. Metally. 1979;(5):11–15. (In Russ.).
53. Vodop’yanov A.G., Kozhevnikov G.N. Dissociation of chromium oxide in the presence of carbon. Metally. 1979;(6): 58–62. (In Russ.).
54. Kosolapova T.Ya., Samsonov G.V. Preparation of higher chromium carbide. Zhurnal prikladnoi khimii. 1959;XXXII(1):55–60. (In Russ.).
55. Zhao Z., Zheng H., Zhang S., Song W., Mao S., Chen Y. Effect of reaction time on phase composition and microstructure of chromium carbide nanopowders. International Journal of Refractory Metals and Hard Materials. 2013;41: 558–562. http://doi.org/10.1016/j.ijrmhm.2013.07.007
56. Eick B.M., Youngblood J.P. Carbothermal reduction of metal-oxide powders by synthetic pitch to carbide and nitride ceramics. Journal of Materials Science. 2009;44:1159–1171. http://doi.org/10.1007/s10853-009-3249-6
57. Zhao Z., Zheng H., Wang Y., Mao S., Niu J., Chen Y., Shang M. Synthesis of chromium carbide (Cr3C2) nanopowders by the carbonization of the precursors. International Journal of Refractory Metals and Hard Materials. 2011;29(5):614–617. http://doi.org/10.1016/j.ijrmhm.2011.04.007
58. Krutskii Yu.L., Dyukova K.D., Bannov A.G., Ukhina A.V., Sokolov V.V., Pichugin A.Yu., Krutskaya T.M., Netskina O.V., Samoilenko V.V. Synthesis of high-dispersion higher chromium carbide powder using nanofibre carbon. Powder Metallurgy and Functional Coatings. 2014;(3):3–8. (In Russ.). http://doi.org/10.17073/1997-308X-2014-3-3-8
59. Krutskii Yu.L., Dyukova K.D., Kuz’min R.I., Maksimovskii E.A., Veselov S.V. Use of carbon material with developed surface for the synthesis of higher chromium carbide. Izvestiya. Ferrous Metallurgy. 2019;62(2):115–122. (In Russ.). http://doi.org/10.17073/0368-0797-2019-2-115-122
60. Kuvshinov G.G., Mogilnykh Yu.L., Kuvshinov D.G., Yermakov D.Yu., Yermakova M.A., Salanov A.N., Rudina N.A. Mechanism of porous filamentous carbon granule formation on catalytic hydrocarbon decomposition. Carbon. 1999;37(8):1239–1246. http://doi.org/10.1016/S0008-6223(98)00320-0
61. Preiss H., Schultze D., Szulzewsky K. Carbothermal synthesis of vanadium and chromium carbides from solution-derived precursors. Journal of the European Ceramic Society. 1999;19(2):187–194. http://doi.org/10.1016/S0955-2219(98)00191-5
62. Zhao Z., Zheng H., Liu S., Shen J., Song W., Chen J. Low temperature synthesis of chromium carbide (Cr3C2) nanopowders by a novel precursor method. International Journal of Refractory Metals and Hard Materials. 2015;48:46–50. http://doi.org/10.1016/j.ijrmhm.2014.07.026
63. Khoshandam B., Kumar R.V. Producing chromium carbide using reduction of chromium oxide with methane. American Institute of Chemical Engineers Journal. 2006;52(3):
64. –1102. http://doi.org/10.1002/aic.10712
65. Wang S.-H., Lin H.-T., Nayak P.K., Chang C.-Y., Huang J.-L. Carbothermal reduction process for synthesis of nanosized chromium carbide via metal-organic vapor deposition. Thin Solid Films. 2010;518(24):7360–7365. http://doi.org/10.1016/j.tsf.2010.05.001
66. Ostrovski O., Guangqing Z. Reduction and carburization of metal oxides by methane-containing gas. American Institute of Chemical Engineers Journal. 2006;52(1):300–310. http://doi.org/10.1002/aic.10628
67. Ebrahimi-Kahrizsangi R., Zadeh H.M., Nemati V. Synthesis of chromium carbide by reduction of chromium oxide with methane. International Journal of Refractory Metals and Hard Materials. 2010;28(3):412–415. http://doi.org/10.1016/J.IJRMHM.2010.01.001
68. Saburov V.P., Cherepanov A.N., Zhukov M.F., Krushenko G.G., Galevskii G.V., Borisov V.T Plasma Chemical Synthesis of Ultra-Dispersed Powders and Their Use for Modifying Metals and Alloys. Vol. 12. Low-Temperature Plasma. Novosibirsk: Nauka, Sibirskaya izdatel’skaya firma RAS; 1995:344. (In Russ.).
69. Krutskii Yu.L., Galevskii G.V., Kornilov A.A. Oxidation of ultra-dispersed powders of boron, vanadium and chromium carbides. Poroshkovaya metallurgiya. 1983;(2):47–50. (In Russ.).
70. Isaeva N.V., Blagoveshchenskii Yu.V., Blagoveshchenskaya N.V., Mel’nik Y.I., Samokhin A.V., Alekseev N.V., Astashov A.G. Production of nanopowders and carbide mixtures using low-temperature plasma. Powder Metallurgy and Functional Coatings. 2013;(3):7–14. (In Russ.).
71. Nozdrin I.V., Shiryaeva L.S., Rudneva V.V. Plasma synthesis and physical and chemical certification of nanochromium carbide. Izvestiya. Ferrous Metallurgy. 2012;55(12):3–8. (In Russ.).
72. Naumenko V.Ya. Production of transition metal carbides of groups IV-V in their homogeneity areas. Poroshkovaya metallurgiya. 1970;10:20–22. (In Russ.).
73. Won H.I., Nersisyan H., Won C.W., Lee H.H. Simple synthesis of nano-sized refractory metal carbides by combustion process. Journal of Materials Science. 2011;46:6000–6006. http://doi.org/10.1007/s10853-011-5562-0
74. Li J., Fu Z.Y., Wang W.M., Wang H., Lee S.H., Niihara K. Preparation of ZrC by self-propagating high-temperature synthesis. Ceramics International. 2010;36(5):1681–1686. http://doi.org/10.1016/j.ceramint.2010.03.013
75. Davoodi D., Hassanzadeh-Tabrizi S.A., Emami A.H., Salanshour S. A low temperature mechanochemical synthesis of nanostructured ZrC powder by a magnesiothermic reaction. Ceramics International. 2015;41(7):8397–8401. http://doi.org/10.1016/j.ceramint.2015.03.034
76. Zhou L., Yang L., Shao L., Chen B., Meng F., Qian Y., Hu L. General fabrication of boride, carbide and nitride nanocrystals via a metal-hydrolysis-assisted process. Inorganic Chemistry. 2017;56(5):2440–2447. http://doi.org/10.1021/acs.inorgchem.6b02501
77. Mandavi M., Ramazani M., Darvishi Z. Investigation of template effect on zirconium carbide synthesis process in carbotherhermal method at low temperature condition. Advanced Powder Technology. 2016;27(4):1547–1551. http://doi.org/10.1016/j.apt.2016.05.016
78. Elyutin V.P., Pavlov J.A., Polyakov V.P., Sheboldaev S.B. Interaction of Metal Oxides with Carbon. Moscow: Metallurgiya; 1976:360. (In Russ.).
79. Stolle S., Gruner W., Pitschke W., Berger L.-M., Wetzig K. Comparative microscale investigations of the carbothermal synthesis of (Ti, Zr, Si) carbides with oxide intermediates of different volatilities. International Journal of Refractory Metals and Hard Materials. 2000;18(1):61–72. http://doi.org/10.1016/S0263-4368(00)00018-4
80. Sondhi A., Morandi C., Reidy R.F., Scharf T.W. Theoretical and experimental investigations on the mechanism of carbothermal reduction of zirconia. Ceramics International. 2013;39(4):4489–4497. https://doi.org/10.1016/j.ceramint.2012.11.043
81. Moiseev G.K., Popov S.K., Ovchinnikov L.A., Vatolin N.A. Formation of titanium and zirconium carbides when their oxides interact with carbon in low-temperature plasma. Izvestiya AN SSSR. Neorganicheskie materialy. 1982;18(9):1521–1524. (In Russ.).
82. Shumilova R.G., Kosolapova T.Ya. Production of zirconium carbide on a semi-industrial scale. Poroshkovaya metallurgiya. 1968;(4):86–89. (In Russ.).
83. Seo M., Kang S., Kim Y., Ryu S.-S. Preparation of highly dispersed ultra-fine ZrC by combination of carbothermal reduction of ball-milled ZrO2 and C mixture and bead milling. International Journal of Refractory Metals and Hard Materials. 2013;41:345–350. http://doi.org/10.1016/j.ijrmhm.2013.05.007
84. Krutskii Yu.L., Dyukova K.D., Bannov A.G., Maksimovskii E.A., Ukhina A.V., Krutskaya T.M., Netskina O.V., Kuznetsova V.V. Synthesis of fine zirconium carbide powder with carbon nanofibers. Analysis and Data Processing Systems. 2015;60(3):192–205. (In Russ.). http://doi.org/10.17212/1814-1196-2015-3-192-205
85. Krutskii Yu.L., Maksimovskii E.A., Popov M.V., Netskina O.V., Cherkasova N.Yu., Kvashina T.S., Chushenkov V.I., Smirnov A.I., Felof’yanova A.V., Aparnev A.I. Synthesis of highly dispersed zirconium carbide. Russian Journal of Applied Chemistry. 2018;91(3):428–435. http://doi.org/10.1134/S107042721803014X
86. Leconte Y., Maskrot H., Combemale L., Herlin-Boime N., Reynaud C. Application of the laser pyrolysis to the synthesis of SiC, TiC and ZrC pre-ceramics nanopowders. Journal of Analytical and Applied Pyrolysis. 2007;79(1-2):465–470. http://doi.org/10.1016/j.jaap.2006.11.009
87. Combemale L., Leconte Y., Portier X., Herlin-Boime N., Reynaud C. Synthesis of nanosized zirconium carbide by laser pyrolysis route. Journal of Alloys and Compounds. 2009;483(1-2):468–472. http://doi.org/10.1016/j.jallcom.2008.07.159
88. Sacks M.D., Wang C.-A., Yang Z., Jain A. Carbothermal reduction synthesis of nanocrystalline zirconium carbide and hafnium carbide powders using solution-derived precursors. Journal of Materials Science. 2004;39:6057–6066. http://doi.org/10.1023/B:JMSC.0000041702.76858.A7
89. Dolle M., Gosset D., Bogicevic C., Carolak F., Simeone D., Baldinozzi G. Synthesis of nanosized zirconium carbide by a sol-gel route. Journal of the European Ceramic Society. 2007;27(4):2061–2067. http://doi.org/10.1016/J.JEURCERAMSOC.2006.06.005
90. Yan Y., Huang Z., Liu X., Jiang D. Carbothermal synthesis of ultra-fine zirconium carbide powders using inorganic precursors via sol-gel method. Journal of Sol-Gel Science and Technology. 2007;44:81–85. http://doi.org/10.1007/s10971-007-1595-x
91. Tao X.Y., Qiu W.F., Li H., Zhao T. One pot synthesis of a soluble polymer for zirconium carbide. Chinese Chemical Letters. 2010;21(5):620–623. http://doi.org/10.1016/j.cclet.2010.01.002
92. Zhao D., Hu H., Zhang C., Zhang Y., Wang J. A simple way to prepare precursors for zirconium carbide. Journal of Materials Science. 2010;45:6401–6405. http://doi.org/10.1007/s10853-010-4722-y
93. Yan C., Liu R., Cao Y., Zhang C., Zhang D. Carbothermal synthesis of submicrometer zirconium carbide from polyzirconoxane and phenolic resin by the facile one-pot reaction. Journal of the American Ceramic Society. 2012;95(11):3366–3369. http://doi.org/10.1111/j.1551-2916.2012.05456.x
94. Ang C., Williams T., Seeber A., Wang H., Cheng Y.-B. Synthesis and evolution of zirconium carbide via sol-gel route: Features of nanoparticle oxide carbon reactions. Journal of the American Ceramic Society. 2013;96(4):1099–1106. http://doi.org/10.1111/jace.12260
95. Yan C., Liu R., Cao Y., Zhang C., Chang D. Synthesis of zirconium carbide powders using chitosan as carbon source. Ceramics International. 2013;39(3):3409–3412. http://doi.org/10.1016/j.ceramint.2012.09.032
96. Chu A., Qin M., Rafi-ud-din, Zhang L., Lu H., Jia B., Qu X. Carbothermal synthesis of ZrC powders using a combustion synthesis precursor. International Journal of Refractory Metals and Hard Materials. 2013;36:204–210. http://doi.org/10.1016/J.IJRMHM.2012.08.016
97. Xie J., Fu Z., Wang Y., Lee S.W., Niihara K. Synthesis of nanosized zirconium carbide powders by a combinational method of sol-gel and pulse current heating. Journal of the European Ceramic Society. 2014;34(1):13e.1–13e.7. http://doi.org/10.1016/j.jeurceramsoc.2013.07.003
98. Wu H., Zhang W., Zhang J. Pyrolysis synthesis and microstructure of zirconium carbide from new preceramic polymers. Ceramics International. 2014;40(4):5967–5972. http://doi.org/10.1016/j.ceramint.2013.11.044
99. Dai L., Wang H., Zhou H., Yu Y., Zhu J., Li Y., Wang L. Direct electrochemical synthesis of zirconium carbide from zirconia. Ceramics International. 2015;41(3):4182–4188. http://doi.org/10.1016/j.ceramint.2014.12.101
100.
101.
102.
103.
About the Authors
Yu. L. KrutskiiRussian Federation
Yurii L. Krutskii, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Chemistry and Chemical Technology”
20 K. Marksa Ave., Novosibirsk 630073, Russian Federation
T. S. Gudyma
Russian Federation
Tat’yana S. Gudyma, Postgraduate of the Chair “Chemistry and Chemical Technology”
20 K. Marksa Ave., Novosibirsk 630073, Russian Federation
T. M. Krutskaya
Russian Federation
Tat’yana M. Krutskaya, Cand. Sci. (Chem.), Assist. Prof. of the Chair of Physics and Chemistry
113 Leningradskaya Str., Novosibirsk 630008, Russian Federation
А. О. Semenov
Russian Federation
Andrei O. Semenov, Senior Lecturer of Division of Nuclear Fuel Cycle of School of Nuclear Science and Engineering
30 Lenina Str., Tomsk 634050, Russian Federation
A. V. Utkin
Russian Federation
Aleksei V. Utkin, Cand. Sci. (Chem.)., Senior Researcher of the Laboratory of Chemical Materials Science
18 Kutateladze Str., Novosibirsk 630090, Russian Federation
Review
For citations:
Krutskii Yu.L., Gudyma T.S., Krutskaya T.M., Semenov А.О., Utkin A.V. Carbides of transition metals: Properties, application and production. Review. Part 2. Chromium and zirconium carbides. Izvestiya. Ferrous Metallurgy. 2023;66(4):445-458. https://doi.org/10.17073/0368-0797-2023-4-445-458
JATS XML