Scroll to:
Hydrometallurgical refining of metallurgical silicon
https://doi.org/10.17073/0368-0797-2023-2-215-221
Abstract
The paper presents the results of refining silicon of metallurgical grades based on leaching of impurities with inorganic acids. Silicon samples were studied by metallographic and X-ray fluorescent methods of analysis, as well as X-ray spectral microanalysis. To improve the quality of this alloying element, we carried out experimental work on its hydrometallurgical purification with solutions of various acids (10 % H2SO4 , HCl, HNO3 , 4 % HF) and their mixtures. Values of changes in the Gibbs energy were calculated for reactions of interaction with reagents of the main impurity inclusions recorded in the studied silicon samples (FeSi2 , Fe2Si, FeSi, AlFeSi, AlFeSi2 , Al3FeSi2 , FeSi2Ti, FeAlTiSi, TiSi2 , Ca2Si). The experiments were carried out on silicon samples with a particle size of –200 μm with constant stirring by a magnetic stirrer at a temperature of 60 °С, duration 1 h and L:S = 5:1. Determination of concentration of the impurity elements in the solution after leaching was made by the atomic emission method of analysis. When hydrofluoric acid is used as a solvent, the best results are obtained for purification of iron, aluminum, and titanium (concentration in solution, mg/dm3, respectively: 2380, 831, 145). The maximum concentration of calcium in the solution (147 mg/dm3 ) was achieved by hydrochloric acid treatment of fine silicon. The most effective for transferring impurities into solution is a mixture of sulfuric and hydrofluoric acids at a ratio of 1:1. Using a mixture of H2SO4 and HCl as a solvent (at a ratio of 1:3) made it possible to achieve sufficiently high mass concentrations of impurity elements in the leaching solution. The degree of silicon purification from iron was 33.32 %, aluminum – 54.64 %, calcium – 65.77 %, titanium – 15.64 %.
Keywords
For citations:
Nemchinova N.V., Tyutrin A.A., Zaitseva A.A. Hydrometallurgical refining of metallurgical silicon. Izvestiya. Ferrous Metallurgy. 2023;66(2):215-221. https://doi.org/10.17073/0368-0797-2023-2-215-221
Введение
Technical (metallurgical) silicon finds widespread application globally in various fields [1]. It is utilized as an alloying element in ferrosilicon and high-silicon alloys [2 – 4], as an alloy in the aluminum industry to produce silumins [5], as a steel deoxidizer in the steel industry [6], and in the chemical industry to obtain organosilanes and other compounds [1; 4]. In the electronics industry, high-purity semiconductor silicon and silicon of “solar” quality serve as the basis for photoelectric current converters [4; 6].
Metallurgical silicon produced by melting in ore thermal furnaces [5; 7 – 9] typically has a purity of only 98.0 – 99.5 %. At present, “solar” quality silicon is manufactured by combining the costly “electronic” silicon and metallurgical silicon, followed by refining through crystallization methods. The Siemens process [10] is the traditional industrial method of producing “electronic” silicon, along with other similar methods that involve vapor phase chemical precipitation for the production of chlorosilane compounds. However, these methods have drawbacks such as high volatility, toxicity, and equipment corrosion in the presence of water. The Siemens process is also highly energy-intensive, consuming approximately 120 kWh/kg of silicon [11]. Alternatively, “solar” quality silicon can be obtained by processing metallurgical silicon, which can involve oxidative refining [5; 12; 13], hydrometallurgical purification [5; 14; 15], vacuum refining [16 – 18], and crystallization methods of purification (direct crystallization, zone melting) [19 – 22]. Of these methods, hydrometallurgical refining is the only process that does not require high temperatures (below 100 °C) or expensive equipment. This method is energy-efficient and cost-effective.
The objective of this study is to conduct experiments for hydrometallurgical purification of metallurgical silicon using various inorganic acids.
Subject of research
The aim of this study was to investigate metallurgical silicon samples obtained from Silicon JSC, RUSAL (Shelekhov, Irkutsk Region) after undergoing oxidative refining.
Technical silicon is typically produced through a continuous method in ore-thermal furnaces (OTF) using silica-containing raw materials with a minimum of 98.5 % SiO2 . Fossil quartzite is a mineral component commonly used in charge mixtures for industrial processes. The mixture typically includes a combination of carbon reducing agents such as charcoal, petroleum coke, and hard coal from various producers such as Kazakhstan and Colombia. Additionally, wood chips are often used as a charge loosener [5; 7].
The process of silicon smelting can be generally described by the reaction SiO2 + 2C = Si + 2CO. However, this oversimplified reaction does not adequately capture the complexity of the reduction of silica that occurs in a furnace during silicon production. In an OTF, silicon production is a highly intricate, high-temperature process that involves various chemical reactions resulting in the formation of intermediate compounds such as SiO and SiC.
The raw materials used for silicon production, such as Cheremshansk Mine quartzite, exhibit heterogeneity in terms of impurity content. As a result, the resulting smelted silicon contains small amounts of iron, calcium, aluminum, and titanium, which give rise to the formation of various intermetallic inclusions within the silicon [23; 24].
At Silicon JSC, the process of silicon production involves the smelting of the charge in an OTF, followed by an oxidative refining step in which air blowing is used to remove mainly aluminum and calcium from the silicon melt [7]. However, this method does not remove iron from the silicon, which highlights the importance of strict control over the supply of iron from the charge materials (quartzite, carbonaceous reducing agents). Alternatively, other methods may be suggested to improve the quality of silicon.
Different methods were employed to study the chemical composition of technical silicon samples used in experimental works.
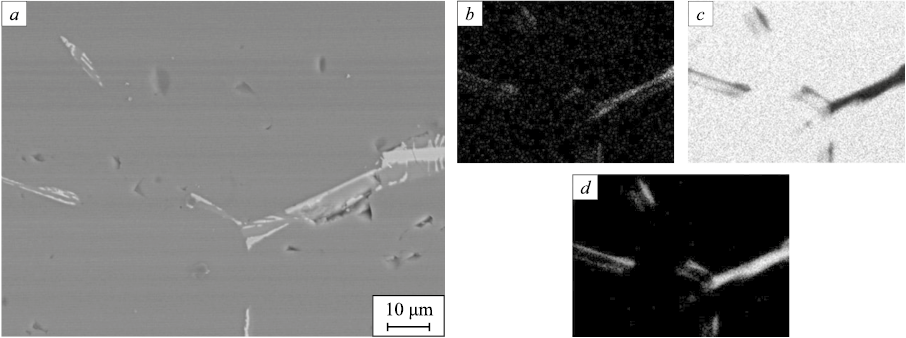
Different methods were employed to study the chemical composition of technical silicon samples used in experimental works. Metallographic studies of thin sections of the initial lump silicon revealed the presence of mainly impurity intermetallic inclusions (Fig. 1). This study was conducted using an Olympus GX-51 metallographic microscope (Olympus, Japan) equipped with an Altera20 digital camera. Additionally, X-ray spectral microanalysis (Fig. 2) was performed using an S4 Pioneer X-ray spectrometer (Bruker, Germany). The results indicated that iron and aluminum were the main impurity components of intermetallic inclusions present in the silicon sample, albeit in insignificant amounts.
Fig. 1. Inclusions of intermetallides in a silicon sample:
Fig. 2. Results of X-ray spectral microanalysis of metallurgical silicon samples (a) |
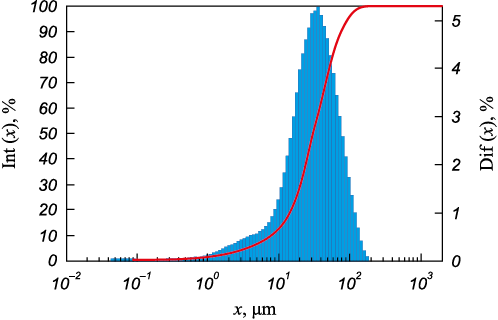
The initial lump material was crushed using a by a ShchD-10 jaw crusher (Russia), and subsequently ground using a ShM-1408 ball mill (Russia). The grinding process employed steel balls without lining, and magnetic separation was then applied. The particle-size distribution of the resulting fine silicon was analyzed using a laser particle size analyzer, Analyzette 22 NanoTecPlus (FRITSCH, Germany). The iron impurity is present in small inclusions (Fig. 2), resulting in a uniform distribution by fractions. The results of the analysis are presented in Fig. 3 and in the Table.
Fig. 3. Particles size distribution in a sample of metallurgical silicon
Results of particle size analysis of powdered metallurgical silicon
| |||||||||||||||||||||||||||||||||||||||||||||
Based on the particle-size analysis, it was found that the metallurgical silicon particles had a fraction size of –200 µm; with 80 % of all particles falling within the fraction size range of +12 ÷ 100 µm. Elemental chemical composition (wt. %) of the material was determined through X-ray fluorescent analysis (XRF), which resulted in the following values (wt. %): Al 0.53; Ti 0.0491; Ca 0.0628; V 0.0066; Cr 0.0024; Mn 0.0145; Fe 0.6094; Cu 0.0037; P 0.0106; Ba 0.0077; Ni 0.0071; Zn 0.0022 and Si 98.6939 (taking into account 12 impurities). The XRF analysis was conducted using an S4 Pioneer X-ray spectrometer.
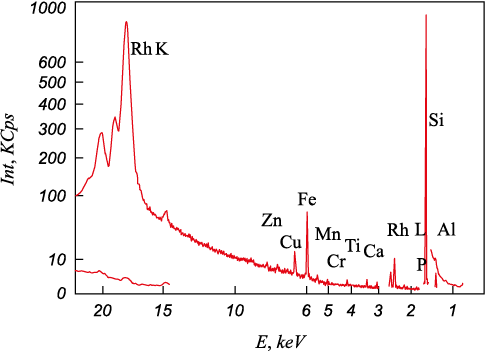
The XRF spectra of the technical silicon samples after oxidative refining (Fig. 4) indicated the presence of various impurity elements such as iron, titanium, aluminum, and calcium. Iron was the most prevalent impurity, and is notoriously difficult to remove through flux-oxygen refining of silicon, primarily due to its low affinity to oxygen. As a result, iron remains almost entirely within the silicon melt and does not transfer to slag.
Fig. 4. X-ray spectrum of a sample of metallurgical silicon |
Experimental work on hydrometallurgical purification of silicon
The quality of technical silicon was attempted to be improved by using solutions of sulfuric, nitric, hydrochloric, and hydrofluoric acids, as well as their mixtures in different proportions as reagents for silicon processing. To assess the potential of these solvents in hydrometallurgical refining, changes in Gibbs energy (Δ\(G^{\circ }_{298}\)) were calculated as an indicator of the thermodynamic likelihood of chemical interactions between impurity inclusions (intermetallics) and various solvents.
Iron impurities in silicon can exist in various forms such as double silicides including (FeSi2 , Fe2Si, FeSi) and in complex intermetallic compounds containing titanium and/or aluminum including (AlFeSi, AlFeSi2 , FeTiSi, FeTiSi2 , FeAl3Si2 , FeAlTiSi) [23 – 25]. The interaction of FeSiTi intermetallic with a sulfuric acid solution can be described by the following reaction
2FeSiTi + 7H2SO4 + 6H2O = Fe2(SO4)3 + 2Ti(SO4)2 + 2H2SiO3 + 11H2
the Δ\(G^{\circ }_{298}\) value is –2412.34 kJ/mol, indicating a spontaneous process.
A software program was developed in Microsoft Excel for the rapid calculation of the Δ\(G^{\circ }_{298}\) values of chemical reactions [26]. The program was used to determine the Δ\(G^{\circ }_{298}\) values for the interactions of various compounds, including FeSi2 , Fe2Si, FeSi, AlFeSi, AlFeSi2 , Al3FeSi2 , FeSi2Ti, FeAlTiSi, TiSi2 and Ca2Si with solutions of different acids. The calculated Δ\(G^{\circ }_{298}\) values were found to be negative [27].
In order to conduct the experiments, silicon samples with a particle size of –200 µm were obtained. Leaching of a 40 g portion of silicon was performed in a 400 mL thermostable beaker for duration of 1 h in a sand bath using a PE6110 magnetic stirrer (100 rpm) with automatic heating. During the experiment, the solution temperature spontaneously reached 60 °C. Acid solutions of varying concentrations, expressed as weight percentages, were employed, including (wt. %): H2SO4 10; HCl 10; HNO3 10; HF 4. These specific values of solvent concentrations were chosen based on the prior research experiences of other scholars [5; 28 – 30]. The liquid to solid ratio was maintained at 5:1 and the required volume of the reagent was calculated taking into account the density of the acid of the set concentration [31].
Results and discussion
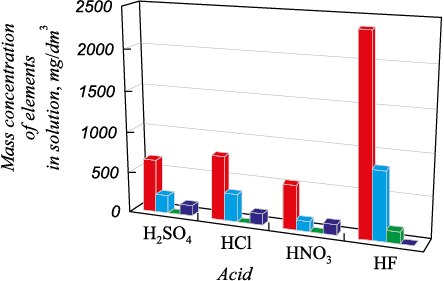
Following acid refining of silicon, the resulting solutions were analyzed for impurity content (Fig. 5) using atomic-emission analysis (AEA) through a PDA-8000 spectrometer (Shimadzu, Japan).
Fig. 5. Comparative histogram of impurities concentration |
The best transfer of impurity elements to the solution was achieved when hydrofluoric acid was used as the solvent, resulting in concentrations of 2380 mg/dm3 of iron, 831 mg/dm3 of aluminum, and 145 mg/dm3 of titanium. The highest concentration of calcium transfer to the leaching solution was obtained using hydrochloric acid (with a concentration of 147 mg/dm3).
These results demonstrate the potential use of the above acids for the deeper purification of metallurgical silicon obtained after oxidative refining at Silicon JSC. Experiments were conducted to leach impurities in the solution using acid mixtures. Ratios of sulfuric, hydrochloric, nitric, and hydrofluoric acids were selected as follows: 1:1, 3:1, and 1:1. The experimental conditions, including temperature, liquid-to-solid (L:S) ratio, stirring speed, and duration, remained unchanged. Fifteen experiments were conducted to leach impurities using various combinations of acid mixtures. The most effective results of impurity transfer to the solution of hydrometallurgical treatment of fine silicon are presented in Figure 6 (AEA data).
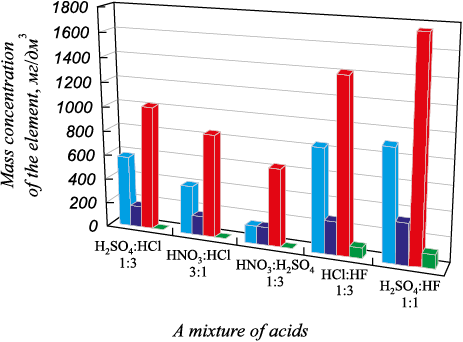
Fig. 6. Results of atomic emission analysis |
The mass concentration of titanium in the leaching solution was 2.77, 2.39 and 1.54 mg/dm3, respectively, when using three acids in different ratios (H2SO4:HCl = 1:3, HNO3:HCl = 3:1, HNO3 :H2SO4 = 1:3).
AEA results of the solutions after silicon hydrometallurgical refining showed that a mixture of sulfuric and hydrofluoric acids in a ratio of 1:1 was the most effective in transferring impurities to the solution. When this acid mixture was used as a solvent, the mass concentration of iron, aluminum, calcium, and titanium in the solution was the highest. The use of a mixture of sulfuric and hydrochloric acids as a solvent in a 1:3 ratio resulted in relatively high mass concentrations of impurity elements in the leaching solution (compared to the use of acids separately, except HF).
Conclusion
In this study, acid treatment was carried out on fine silicon samples to purify metallurgical silicon. Reagents such as 10 % solutions of hydrochloric, sulfuric, and nitric acids, as well as 4 % hydrofluoric acid, were used. These solvents were thermodynamically capable of interacting with impurity metal-containing compounds in silicon.
The use of hydrofluoric acid as a solvent resulted in the highest transfer of iron, aluminum, and titanium to the solution. Hydrochloric acid was found to be the most effective for calcium transfer. Furthermore, a mixture of H2SO4 and HCl at a ratio of 1:3 produced rather high mass concentrations of impurity elements in the leaching solution. The degree of iron removal was 33.32 %, aluminum – 54.64 %, calcium – 65.77 %, and titanium – 15.64 %. When a mixture of acids was used, a 1:1 ratio of sulfuric and hydrofluoric acids was found to be the most effective for maximum transfer of impurities to the solution, with the highest mass concentration of iron, aluminum, calcium, and titanium. The degree of removal for iron was 88.37 %, aluminum – 81.85 %, calcium – 94.62 % and titanium – 92.22 %. From an industrial standpoint, it is more economically and ecologically feasible to choose a mixture of 10 % sulfuric and hydrochloric acids at a 1:3 ratio for silicon purification.
References
1. Jorn P. Silicon in the 2020s. In: Silicon for the Chemical and Solar Industry XV. Proceeding of the Int. Conf. June 15 – 18. Norway, Trondheim, 2020:57–64.
2. Holappa L. Toward sustainability in ferroalloys production. In: Proceeding of the Twelfth Int. Ferroalloys Congress. June 6 – 9. Finland, Helsinki, 2010:1–10.
3. Schei A., Tuset J.Kr., Tveit Н. Production of High Silicon Alloys. Trondheim: Tapir; 1998:363.
4. Rozhikhina I.D., Nokhrina O.I., Elkin K.S., Golodova M.A. Ferroalloy production: State and trends of development in the world and Russia. In: Metallurgy – 2019: Technologies, Innovations, Quality. Part 1. Proceedings of the XXI Int. Sci. and Pract. Conf., November 23–24, 2019. Novokuznetsk: ITs SibSIU; 2019:20–32. (In Russ.).
5. Popov S.I. Metallurgy of Silicon in Three-Phase Ore-Thermal Furnaces. Irkutsk: Kremnii; 2004:237. (In Russ.).
6. Gasik M.I., Gasik M.M. Silicon Electrothermy. Dnepropetrovsk: Natsional’naya metallurgicheskaya akademiya Ukrainy; 2011:487. (In Russ.).
7. Katkov O.M. Smelting of Technical Silicon. Irkutsk: IrSTU; 1999:243. (In Russ.).
8. Ringdalen E., Tangstad M. Reaction mechanisms in carbothermic production of silicon, study of selected reactions. In: Int. Smelting Technology Symposium 2012. Downey J.P., Battle Th.P. eds. 2012:195–203. https://doi.org/10.1002/9781118364765.ch24
9. Gasik M. Handbook of Ferroalloys: Theory and Technology. Oxford: Butterworth-Heinemann; 2013:536.
10. Dazhou Y. Siemens Process. In: Handbook of Photovoltaic Silicon. Berlin, Heidelberg: Springer; 2017:1–32. https://doi.org/10.1007/978-3-662-52735-1_4-1
11. Braga A.F.B., Moreira S.P., Zampieri P.R., Bacchin J.M.G., Mei P.R. New processes for the production of solar-grade polycrystalline silicon: A review. Solar Energy Materials and Solar Cells. 2008;92(4):418–424. https://doi.org/10.1016/j.solmat.2007.10.003
12. Johnston M., Barati M. Distribution of impurity elements in slag-silicon equilibria for oxidative refining of metallurgical silicon for solar cell applications. Solar Energy Materials and Solar Cells. 2010;94(12):2085–2090. https://doi.org/10.1016/j.solmat.2010.06.025
13. Nepomnyashchikh A.I., Gribov B.G., Eliseev I.A., Kalashnik O.N. Unconventional silicon production technologies for solar energy. In: Abstracts of the VI Int. Conf. and the V School of Young Scientists and Specialists on Topical Issues of Physics, Materials Science, Technology and Diagnostics of Silicon, Nanometer Structures and Devices based on it “Silicon-2009”, July 7–10, 2009. Novosibirsk: Nikolaev Institute of Inorganic Chemistry SB RAS, 2009:26. (In Russ.).
14. Xi F., Li Sh., Ma W., Chen Zh., Wei K., Wu J. A review of hydrometallurgy techniques for the removal of impurities from metallurgical-grade silicon. Hydrometallurgy. 2021; 201:105553. https://doi.org/10.1016/j.hydromet.2021.105553
15. Nemchinova N.V., Tyutrin A.A. Study of the process of silicon hydrometallurgical refining of. In: Non-Ferrous Metals-2011. Materials of the 3rd Int. Congress, 7-9 September 2011, Krasnoyarsk. 2011:342–344. (In Russ.).
16. Wei K., Zheng D., Ma W., Yang B., Dai Y. Study on Al removal from Mg–Si by vacuum refining. Silicon. 2015; 7(3):269–274. https://doi.org/10.1007/s12633-014-9228-9
17. Mitrašinović A.M., Souza R.D., Utigard T.A. Impurity removal and overall rate constant during low pressure treatment of liquid silicon. Journal of Materials Processing Technology. 2012;212(1):78–82. https://doi.org/10.1016/j.jmatprotec.2011.08.006
18. Kravtsov A.A. Method of Silicon Vacuum Cleaning. Patent no. 2381990 RF; Publ. 20.02.2010. (In Russ.).
19. Tanay F., Dubois S., Enjalbert N., Veirman J. Low temperature-coefficient for solar cells processed from solar-grade silicon purified by metallurgical route. Progress in Photovoltaics: Research and Applications. 2011;19(8):966–972. https://doi.org/10.1002/pip.1104
20. Jiang D., Tan Y., Shi S., Dong W., Gu Z., Zou R. Removal of phosphorus in molten silicon by electron beam candle melting. Materials Letters. 2012;78:4–7. https://doi.org/10.1016/j.matlet.2012.03.031
21. Nepomnyashchikh A.I., Krasin B.A., Vasil’eva I.E., Eliseev I.A., Eremin V.P., Fedosenko V.A., Sinitskii V.V. Silicon for solar energy. Izvestiya Tomskogo politekhnicheskogo universiteta. 2000;300(2):176–190. (In Russ.).
22. Leblanc D., Boisvert R. Process and apparatus for purifying silicon. In.: Silicon for the Chemical аnd Solar Industry IX: Proceedings of the Int. Sci. Conf. June 23–26. Norway, Oslo. 2008:121–129.
23. Nemchinova N.V. Investigation of chemical composition of metallurgical silicon. Izvestiya vuzov. Prikladnaya khimiya i biotekhnologiya. 2012;(1(2)):129–134. (In Russ.).
24. Nemchinova N.V., Hoang V.V., Tyutrin A.A. Formation of impurity inclusions in silicon when smelting in ore-thermal furnaces. IOP Conference Series: Materials Science and Engineering. 2020;969:012038. https://doi.org/10.1088/1757-899X/969/1/012038
25. Nemchinova N.V. Behavior of Impurity Elements in Production and Refining of Silicon. Moscow: Akademiya estestvoznaniya; 2008:237. (In Rus.).
26. Nemchinova N.V., Zaitseva A.A. Calculation of thermodynamic probability of chemical reactions during acid refining of metallurgical silicon. Certificate no. 2022612200 RF; Publ. 08.02.2022. (In Russ.).
27. Zaitseva A.A., Zyryanov N.V. Possibility of using various acids in hydrometallurgical refining of metallurgical silicon. In: Prospects for the Development of Technology for Processing Hydrocarbon and Mineral Resources. Materials of XII All-Russ. Sci. and Pract. Conf. with International Participation. Irkutsk: INRTU, 2022:88–91. (In Russ.).
28. Zadde V.V., Lesnikov A.K., Lesnikov P.A., Strebkov D.S. Method of purification of powdered silicon. Patent no. 2388691 RF; Publ. 10.11.2009. (In Russ.).
29. Nemchinova N.V., Tyutrin A.A., Zelinskaya E.V. Acidic-ultrasonic refining of silicon by carbothermic technology. Metallurgist. 2015;59(3):258–263. https://doi.org/10.1007/s11015-015-0094-5
30. Tamendarov M.F., Chumikov G.N., Tokmoldin S.Zh. Investigation of phosphoric slags for obtaining solar-quality silicon. In: Chemistry and Metallurgy of Complex Processing of Mineral Raw Materials: Materials of Int. Sci. and Pract. Conf., June 25–26, 2015. Kazakhstan, Karaganda. 2015: 115–120. (In Russ.).
31. Rabinovich V.A., Khavin Z.Ya. Brief Chemical Guide. Leningrad: Khimiya; 1978:269. (In Russ.).
About the Authors
N. V. NemchinovaRussian Federation
Nina V. Nemchinova, Dr. Sci. (Eng.), Prof., Head of the Chair “Non-Ferrous Metallurgy”
83 Lermontova Str., Irkutsk 664074, Russian Federation
A. A. Tyutrin
Russian Federation
Andrei A. Tyutrin, Cand. Sci. (Eng.), Assist. Prof. of the Chair “Non-Ferrous Metallurgy”
83 Lermontova Str., Irkutsk 664074, Russian Federation
A. A. Zaitseva
Russian Federation
Anna A. Zaitseva, Postgraduate, Assistant of the Chair “Non-Ferrous Metallurgy”
83 Lermontova Str., Irkutsk 664074, Russian Federation
Review
For citations:
Nemchinova N.V., Tyutrin A.A., Zaitseva A.A. Hydrometallurgical refining of metallurgical silicon. Izvestiya. Ferrous Metallurgy. 2023;66(2):215-221. https://doi.org/10.17073/0368-0797-2023-2-215-221