Scroll to:
Effect of B\(_{2}\)O\(_{3}\) on viscosity of high-magnesia blast furnace slag
https://doi.org/10.17073/0368-0797-2023-1-89-96
Abstract
Smelters in the Urals procure only 50 – 60 % of raw materials from local sources. The rest is imported from Central Russia, the Kola Peninsula, and Kazakhstan. Switching to local raw materials would increase the competitiveness of the Urals metals, so local alternatives should be considered, such as siderite ore from the Bakal deposit. The ore is in low demand due to its low iron content and high magnesium content. The higher the siderite content in the charge, the higher the magnesium oxide content in the slag. This affects the slag viscosity, so for siderite content exceeding 20%, melting is difficult or impossible. We proposed the addition of boric oxide to liquefy the slag. The simulated slag (CaO 26.8 %; SiO2 38.1 %; Al2O3 11.8 %; MgO 23.6 %) identical to that produced by the Magnitogorsk Metallurgical Plant (MMK) blast furnaces with the addition of 30 % of calcined siderite is short and unstable. The temperature when the slag viscosity is equal to that at the blast furnace taphole (0.5 Pa·s) is 1390 °C, while the melting point (2.5 Pa·s viscosity) is 1367 °C. The addition of boric anhydride makes the slag long and stable. As the B2O3 content is increased from 0 to 12 %, the temperatures at which the slag viscosity is 0.5 and 2.5 Pa·s decrease to 1260 and 1100 °C, respectively. The study shows it is possible to significantly increase the siderite content in blast furnace charge.
Keywords
For citations:
Vusikhis A.S., Leont’ev L.I., Gulyaeva R.I., Sergeeva S.V., Tyushnyakov S.N. Effect of B\(_{2}\)O\(_{3}\) on viscosity of high-magnesia blast furnace slag. Izvestiya. Ferrous Metallurgy. 2023;66(1):89-96. https://doi.org/10.17073/0368-0797-2023-1-89-96
Smelters in the Urals procure only 50 – 60 % of the raw materials from local sources. The rest comes from the central and northwestern regions of Russia and Kazakhstan, since less than half of the 50 iron ore deposits in the Urals region are in operation [1 – 5], and the production rate does not match demand. There are several reasons for that. The siderite iron ore extraction volume at the Bakal deposit (Southern Urals) with its about 1 bln. ton reserves are far less than the deposit capacity due to low demand for low-grade ore. The magnesium oxide content in the mining waste is about 40 – 50 % [6 – 8]. For this reason, siderites can be used only as additives to blast furnace charges or sinter cakes. Bakal siderite is unsuitable as the key component of the blast furnace charge, since the slag will have a very high melting point [9].
Slags are melted in a certain temperature range, so the melting point (Tmp ) is not constant. It is either the liquidus temperature (Tl ) above which the slag is completely liquid or the temperature at which the slag begins to freely flow from the coke packing (the required viscosity should be less than 2.5 Pa∙s).
Also, the slag melting point should be below 1400 °C for smooth, safe smelting, and the slag should have sufficient mobility in the 1400 to 1500 °C temperature range [10 – 13].
The viscosity of acidic slags grows slowly over a relatively wide range of temperatures. That is why such slags are called “long slags”. Basic slags become thicker as the temperature drops below the crystallization point due to heterogenization and solid phase formation. The thickening occurs in a narrow temperature range. Such slags are called “short slags”.
Both slag temperature and fluidity are important variables in the blast furnace smelting process. Indeed, the viscosity of melted slag is the key property affecting blast furnace stability and efficiency and the entire smelting process. Many researchers [14 – 21] have studied the correlation between the slag component (e.g, magnesium oxide) content, melting point, and viscosity. Authors [14 – 16] studied the effects of slag composition on its properties over a wide range of component contents. The results are in good agreement with the studies of a narrower range of contents [17 – 21].
Any blast furnace slag consists of four basic components: CaO – SiO2 – MgO – Al2O3 . For such melts containing less than 15 % of alumina, an increase in the base-to-silica ratio (R) from 0.6 to 1.5, and the magnesium oxide content from 0 to 20 % leads to the melting point increase to 1350 – 1400 °C and narrowing the solidification temperature range. The slags become “shorter”. Any amount of magnesium oxide can be added. Slags containing more than 25 % MgO are not flowable below 1400 °C.
Raising the MgO content from 0 to 25 % in the slag with 0.6 – 1.5 the base-to-silica ratio results in a viscosity drop to a minimum. The minimum value depends on the alumina content and temperature. Acidic slags show higher viscosity drop rates than basic ones.
Slags containing 5 % of Al2O3 have a minimum viscosity (0.15 Pa∙s) at 1500 °C; R ~ 0.9 – 1.1; 17 – 20 % MgO; 36 – 38 % SiO2 . Reducing the temperature to 1400 °C increases the minimum viscosity to 0.35 Pa∙s. Now the minimum viscosity exists in a wider MgO content range of up to 13 – 20 % with a shift towards more acidic slags containing 39 – 41 % of SiO2 .
The alumina content increase to 10 % increases the minimum viscosity. As the temperature drops from 1500 to 1400 °C, the minimum viscosity increases from 0.2 to 0.3 Pa∙s. The composition ranges where the minimum viscosity occurs are narrowed from R ~ 0.8 – 1.2; 13 – 24 % MgO; 35 – 40 % SiO2 (at 1500 °C) to R ~ 1.05 – 1.2; 14 – 16 % MgO; 39 – 41 % SiO2 (1400 °C), respectively.
For the 15 % Al2O3 content, the minimum viscosity increases from 0.30 to 0.55 Pa∙s. The content ranges change from R ~ 0.9 – 1.2; 15 – 26 % MgO; 30 – 33 % SiO2 to R ~ 0.80 – 1.05; 18 – 22 % MgO, 33 – 35 % SiO2 , as the temperature drops from 1500 to 1400 °C. The higher magnesium oxide content leads to a dramatic viscosity reduction in acid slags containing 25 – 35 % of CaO. Such slags with R ~ 0.5 – 0.8, containing 13 – 18 % Al2O3 and 16 – 25 % MgO, are quite fluid at 1350 – 1400 °C.
The melting point of slags containing 20 % Al2O3 (R ~ 1.2 – 1.5) exceeds 1500 °C for any magnesium oxide content. When R ~ 1.1 – 1.2, the crystallization occurs at >16 % MgO. As R decreases to 0.6, the critical content of magnesium oxide increases to 20 %. When MgO/ Al2O3 ~ 0.5, for R ~ 1.1 – 1.2 Тl is about 1450 °C. As R decreases to 0.6, Тl drops to 1350 °C. the minimum viscosity of such slags varies from 0.4 Pa∙s (at 1500 °C) to 1.0 Pa∙s (at 1400 °C) for 34 – 36 % SiO2 content.
The above data indicates that in slags with less than 1.0 MgO base-to-silica ratio, the MgO content can reach 15 – 20 %, with no significant smelting issues. Such slags are sufficiently fluid. They melt at temperatures below 1350 °C. When the MgO content is above 25 %, the melting point raises drastically making the slag short and unstable. The analysis [22] shows that such slags are formed when the blast furnace charge contains about 30 % of siderite. The conclusion is that such a charge is difficult or impossible to use.
As it is known [23 – 25], the addition of boric oxide to blast furnace slag reduces its viscosity over the entire temperature range and makes slags longer.
The purpose of this study is to identify the effect of adding boric oxide on the viscosity and melting point of high-magnesia blast furnace slags.
We produced simulated slag containing 26.8 % CaO, 38.1 % SiO2 , 11.8 % Al2O3 and 23.6 % MgO. Its composition was similar to the estimated [22] composition of the slag produced by blast furnace No. 9, MMK when the charge was a mixture of in-house sinter and pellets from Sokolovo-Sarbai Mining and Concentrating Facility in the 2:1 ratio, and 30 % of calcined siderite concentrate.
The calcium oxide (AR grade) we used was calcinated in a muffle furnace at 910 °C for 6 h. The boric anhydride (B2O3 ) was calcinated at 170 °C for 2 h. The latter was subsequently melted in a resistance furnace at 900 °C for 4 h.
The samples were prepared by heating and melting an oxide mixture (CaO – SiO2 – MgO – Al2O3 ) in a graphite pot at 1500 – 1550 °C (30 min holding time). The melt was poured into the mold and cooled.
After cooling the material was crushed and mixed with boric anhydride to achieve 3, 6, 9, and 12 % B2O3 content. Then the sample was placed in a molybdenum pot, heated to 1550 °C, and its viscosity was measured. We used a forced-oscillation vibrating viscometer [26, 27]. The melt temperature was measured with a tungsten-rhenium thermocouple. The probe was made of molybdenum, in order to avoid its reaction with the melt. The cooling rate was 5 – 7 °C/min.
For combined thermogravimetry and differential scanning calorimetry (DSC), we used a Netzsch STA 449C Jupiter simultaneous thermal analyzer. The experimental data was processed using NETZSCH Proteus Thermal Analysis [28] in its standard configuration (±3 °C temperature accuracy). The samples were heated to 1430 °C and then cooled to 500 °C at the 20 °C/min rate in pure blanketing argon (99.998 % Ar). The pots were made of Pt-Rh; the lids and inserts were made of aluminum oxide. The samples weighing 23 – 30 mg were made from crushed, pre-sintered slag. The sample slags contained the key components (SiO2 – CaO – MgO – Al2O3 ), and 0.6 and 12 % of boric oxide.
We used an XRD-7000 Maxima (Shimadzu) diffractometer (CuKα radiation). The 2θ scattering angle range was 15 – 65°.
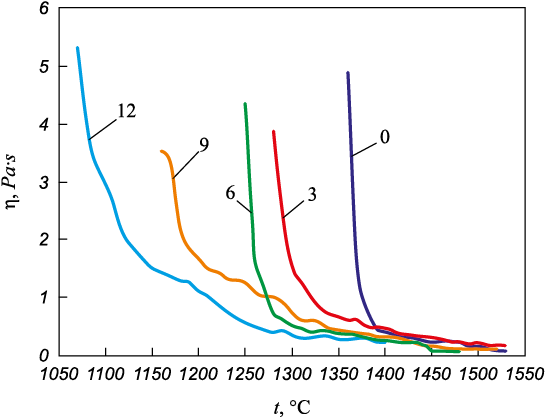
Data analysis (Fig. 1) resulted in the following findings. The slag viscosity vs. temperature curve is similar to the polytherm of a similar slag presented in [15]. The slag viscosity is less than 0.5 Pa∙s at temperatures above 1390 °C. The viscosity increases to 2.5 Pa∙s at about 1370 °C (Tmp ). After that, the slag thickening rate rises sharply. Adding boric anhydride reduces the temperature at which the slag viscosity is less than 0.5 Pa∙s. The slag thickening temperature range is extended to mp . The higher the boric oxide content, the lower the solidification temperature.
Fig. 1. Viscosity polyterms for CaO – SiO2 – MgO – Al2O3 – B2O3 |
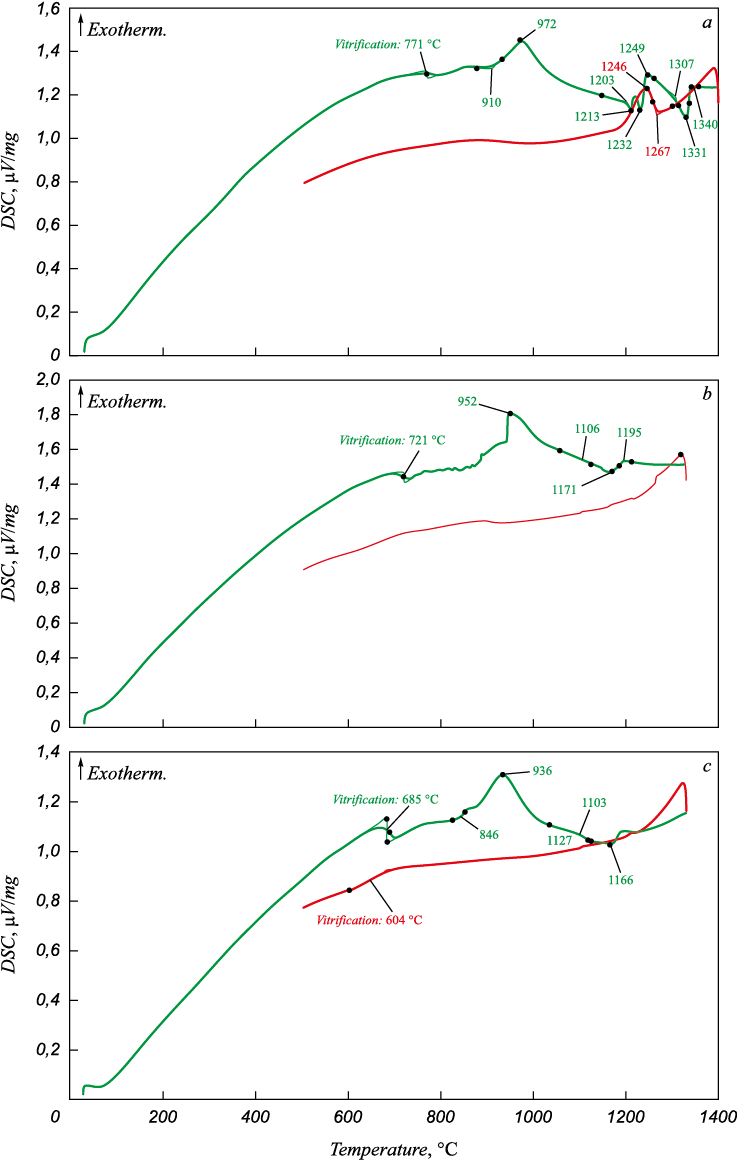
The thermal analysis results are slightly different from the viscosity measurements (Fig. 2). As sample No. 1 (Fig. 2, a) without any B2O3 was heated, the DSC curve indicates devitrification at 771 °C, as well as the exothermic effect of “cold” crystallization beginning at 910 °C and reaching the maximum at 972 °C. It also shows three endothermic effects with their maximums at 1213, 1232, and 1331 °C, apparently caused by the melting of the slag phase components. The liquidus temperature was 1340 °C. The DSC cooling curve shows an exothermic effect of the slag crystallization with its beginning/maximum at 1267/1246 °C.
Fig. 2. DSC curves for heating and cooling of the SiO2 – CaO – MgO – Al2O3 slag samples (a) |
The thermal analysis of sample No. 2 (Fig. 2, b) containing 6 % B2O3 showed the devitrification effect at 721 °C, and the effects of “cold” crystallization (952 °C) and melting (1106/1171 °C). The slag liquidus temperature was 1195 °C. As the slag was cooled, the DSC curve did not show any effects which indicates the slag's amorphous structure is preserved.
Increasing the B2O3 content to 12 % (Fig. 2, c) does not significantly change the DSC curves. We found a slight decrease in the devitrification temperature (tg = 685 °C) during heating, and vitrification effects during cooling (604 °C). The effects of “cold” crystallization and melting were observed at 936 °C and 1103/1166 °C. It is slightly lower than the temperatures found in samples No. 1 and 2.
Generally, the addition of B2O3 to a SiO2 – CaO – MgO – Al2O3 slag reduces the devitrification, “cold” crystallization, and melting temperatures and facilitates the formation and stabilization of the amorphous phase.
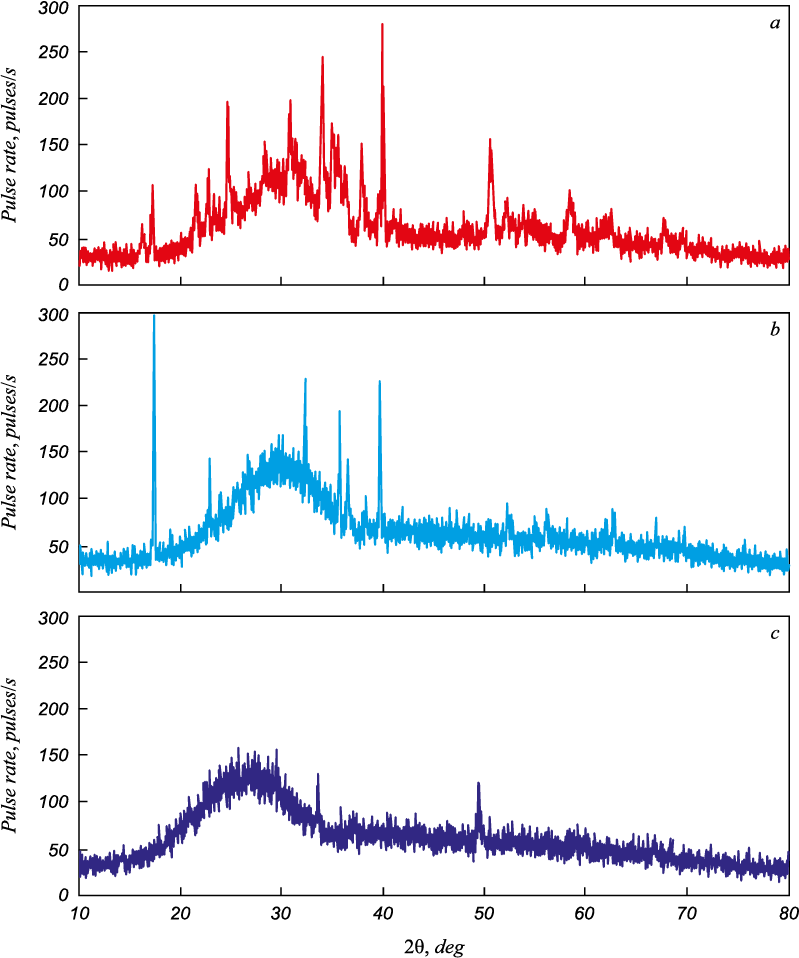
This was also confirmed by the X-ray phase analysis (Fig. 3). Without boric anhydride when the slag is cooled, it crystallizes, and calcium and magnesium aluminosilicates are formed. When boric anhydride is added during cooling, slag vitrification occurs. As a result, the borate component is added to the aluminosilicates.
Fig. 3. Diffractograms of the of SiO2 – CaO – MgO – Al2O3 slag samples (a) |
Conclusions
Siderites (as raw ore, after calcination and concentration, or pelleting) are currently used as additives to blast furnace charges. Their content in the charge is selected in such a way that the magnesium oxide content in the resulting slag does not exceed 15 – 20 %. Such slags are liquid above 1400 °C. Further magnesium oxide content increases, making the slag short and refractory. Melting a charge containing more than 30 % of siderite, which results in a high-magnesia slag (>25 % MgO), is difficult. The addition of boric anhydride to the charge reduces the melting temperature of the slag. For a melt with a 23.6 % magnesium oxide content, adding 0 to 12 % of boric anhydride reduces the temperature at which the slag viscosity is 0.5 Pa∙s from 1390 to 1260 °C, and from 1367 to 1100 °C for the 2.5 Pa∙s viscosity. This makes it possible to significantly increase the siderite content in the charge.
References
1. Volkov Yu.V., Sokolov I.V., Smirnov A.A. Strategy for development of raw material resources in the Urals. Gornaya promyshlennost’. 2006: (4): 57–62. (In Russ.).
2. Volkov Yu.V., Slavikovskii O.V., Sokolov I.V., Smirnov A.A. Prospects for development of raw material base of mining and metallurgical enterprises in the Urals. Gornyi informatsionno-analiticheskii byulleten’. 2007; (5): 286–290. (In Russ.).
3. Pakhomov V.P., Dushin A.V. Analysis of the mineral-raw material safety in the Ural Federation District. Ekonomika regiona. 2008; (3): 129–143. (In Russ.).
4. Valiev N.G., Slavikovskii O.V., Slavikovskaya Yu.O. Peculiarities of development of mineral resource base in the Urals urbanised territories. Gornyi informatsionno-analiticheskii byulleten’ (nauchno-tekhnicheskii zhurnal). 2012; (6):
5. –347. (In Russ.).
6. Kornilkov S.V., Kantemirov V.D. Iron ore deposits of the Nether-Polar Urals as a prospective raw materials base of the Urals metallurgy. Izvestiya vuzov. Gornyi zhurnal. 2015; (8): 22–28. (In Russ.).
7. Krasnoborov V.A., Yaroshevskii S.L., Denisov A.A., Rudin V.S., Biryuchev V.I., Polushkin M.F. Efficiency and Prospects of Using Siderite Ores in Blast Furnace Smelting. Donetsk; 1996: 88. (In Russ.).
8. Yur’ev B.P., Melamud S.G., Spirin N.A., Shatsillo V.V. Technological and Thermal Engineering Bases of Siderite Ore Preparation for Metallurgical Processing: Monograph. Yekaterinburg: Den’ RA; 2016: 428. (In Russ.).
9. Vusikhis A.S., Leont’ev L.I. The Use of Siderite Ores in Production of Iron and Steel: Monograph. Moscow, Vologda: Infra-Inzheneriya; 2022: 116. (In Russ.).
10. Slag Atlas. Düsseldorf, Verlag Stahlissen GmbH; 1995: 616.
11. Gotlib A.D. Blast Furnace Process. Moscow: Metallurgiya; 1966: 503. (In Russ.).
12. Efimenko G.G., Gimmel’farb A.A., Levchenko V.E. Ironmaking. Kiev: Vyshcha shkola; 1988: 351. (In Russ.).
13. Vegman E.F., Zherebin B.N., Pokhvisnev A.N., etc. Ironmaking. Moscow: Akademkniga; 2004: 774. (In Russ.).
14. Badich A., Senk D., Gudenau H.W., Mavrommatis K.Th. Ironmaking. Aahen, RWTH Aahen University; 2008: 402.
15. Bol’shakova L.I., Zhilo N.L. Physical properties of high-magnesia blast furnace slags during smelting of Bakal siderites. In: Slag Mode in Blast Furnaces. Moscow: Metallurgiya; 1967: 173–185. (In Russ.).
16. Zhilo N.L. Formation and Properties of Blast Furnace Slag. Moscow: Metallurgiya; 1974: 120. (In Russ.).
17. Voskoboinikov V.G., Dunaev N.E., Mikhalevich A.G., etc. Properties of Liquid Blast Furnace Slags: Manual. Moscow: Metallurgiya; 1975: 182. (In Russ.).
18. Saito N., Hori N., Nakashima K., Mori K. Viscosity of blast furnace type slags. Metallurgical and Materials Transactions B. 2003; 34(5): 509–516. https://doi.org/10.1007/s11663-003-0018-9
19. Kou M., Wu S., Ma X., Wang L., Chen M., Cai Q., Zhao B. Phase equilibrium studies of CaO–SiO2–MgO–Al2O3 system with binary basicity of 1.5 related to blast furnace slag. Metallurgical and Materials Transactions B. 2016; 47(2): 1093–1102. https://doi.org/10.1007/s11663-016-0584-2
20. Liu Y., Lu X.W., Li B., Bai C.G. Relationship between structure and viscosity of CaO–SiO2–MgO–30.00 wt.% Al2O3 slag by molecular dynamics simulation with FT-IR and Raman spectroscopy. Ironmaking & Steelmaking. 2018; 45(6): 492–501. https://doi.org/10.1080/03019233.2017.1288309
21. Shen F., Hu X., Zheng H., Jiang X., Gao Q., Han H., Long F. Proper MgO/Al2O3 ratio in blast-furnace slag: Analysis of proper MgO/Al2O3 ratio based on observed data. Metals. 2020; 10(6): 784. https://doi.org/10.3390/met10060784
22. Das K., Agrawal A., Reddy A.S., Ramna R.V. FactSage studies to identify the optimum slag regime for blast furnace operation. Transactions of the Indian Institute of Metals. 2021; 74: 419–428. https://doi.org/10.1007/s12666-020-02144-y
23. Vusikhis A.S., Leont’ev L.I., Agafonov S.N. Assessment of the efficiency of the use of Bakal siderites in blast furnace smelting. Izvestiya. Ferrous Metallurgy. 2022; 65(7): 504–510. (In Russ.). https://doi.org/10.17073/0368-0797-2022-7-504-510
24. Ren S., Zhang J., Wu L. Influence of B2O3 on viscosity of high Ti-bearing blast furnace slag. ISIJ International. 2012; 52(6): 984–991. https://doi.org/10.2355/isijinternational.52.984
25. Kim G.H., Sohn I. Role of B2O3 on the viscosity and structure in the CaO–Al2O3–Na2O-based system. Metallurgical and Materials Transactions B. 2014; 45(2): 86–95. https://doi.org/10.1007/s11663-013-9953-2
26. Wang G., Wang J.-S., Xue Q.-G. Properties of boron-rich slag separated from boron-bearing iron concentrate. Journal of Central South University. 2018; 25(4): 783–794. https://doi.org/10.1007/s11771-018-3783-y
27. Selivanov E.N., Gulyaeva R.I., Istomin S.A., Belyaev V., Tyushnyakov S., Bykov A. Viscosity and thermal properties of slag in the process of autogenous smelting of copper–zinc concentrates. Mineral Processing and Extractive Metallurgy. 2015; 124(2): 88–95. https://doi.org/10.1179/1743285514Y.0000000078
28. Vusikhis A.S., Selivanov E.N., Dmitriev A.N., Chentsov V.P., Ryabov V.V. Structure sensitive properties of system B2O3–CaO melts. Defect and Diffusion Forum. 2020; 400: 186–192.
29. https://doi.org/10.4028/www.scientific.net/DDF.400.186
30. NETZSCH Proteus Software. Thermal Analysis. Version 4.8.3.
About the Authors
A. S. VusikhisRussian Federation
Aleksandr S. Vusikhis, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
L. I. Leont’ev
Russian Federation
Leopol’d I. Leont’ev, Academician, Adviser, Russian Academy of Sciences, Dr. Sci. (Eng.), Prof., National University of Science and Technology “MISIS”, Chief Researcher, Institute of Metallurgy, Ural Branch of the Russian Academy of Science
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
4 Leninskii Ave., Moscow 119049, Russian Federation
32a Leninskii Ave., Moscow 119991, Russian Federation
R. I. Gulyaeva
Russian Federation
Roza I. Gulyaeva, Cand. Sci. (Chem.), Senior Researcher of the Laboratory of Pyrometallurgy of Nonferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
S. V. Sergeeva
Russian Federation
Svetlana V. Sergeeva, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Nonferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
S. N. Tyushnyakov
Russian Federation
Stanislav N. Tyushnyakov, Cand. Sci. (Eng.), Senior Researcher of the Laboratory of Pyrometallurgy of Non-Ferrous Metals
101 Amundsena Str., Yekaterinburg 620016, Russian Federation
Review
For citations:
Vusikhis A.S., Leont’ev L.I., Gulyaeva R.I., Sergeeva S.V., Tyushnyakov S.N. Effect of B\(_{2}\)O\(_{3}\) on viscosity of high-magnesia blast furnace slag. Izvestiya. Ferrous Metallurgy. 2023;66(1):89-96. https://doi.org/10.17073/0368-0797-2023-1-89-96