Scroll to:
Influence of silicon carbides on the structure and properties of nickel-phosphorus composite coatings
https://doi.org/10.17073/0368-0797-2023-1-43-49
Abstract
The authors studied the structure, properties, and corrosion resistance in different acids of the nickel-phosphorus coatings with the dispersed silicon carbides after crystallization annealing in different modes. Crystallization onset temperatures after heating at rates of 1, 5, and 20 °C/min and the percentage of crystalline phases formed under isothermal conditions (nickel phosphide Ni3P and nickel) were determined. It was determined that a high microhardness of more than 1000 HV is achieved in the composite nickel-phosphorus coating with dispersed particles of the silicon carbides also during prolonged low-temperature annealing, accompanied by crystallization with the formation of already insignificant (10 %) amounts of Ni3P. The revealed dispersed Ni3P located both inside the grains and along the boundaries of the grains make the main contribution to the increase in microhardness. Yield strength and tensile strength of coatings increase during crystallization annealing by only 12 – 15 MPa, and elongation drops to zero, due to the formation of the brittle Ni3P compounds. Annealing with a short-term soaking at crystallization temperatures leads to the fact that the silicon carbides exhibit a barrier effect. This reduces the intensity of the formation of crystalline Ni3P and corrosion resistance, while a long-term soaking at lower crystallization temperatures forms about 70 % Ni3P, contributing to consistently high hardness and improved corrosion resistance. Corrosion resistance of the composite Ni-P coatings with the silicon carbides, regardless of heat treatment modes, is maximum in acetic and orthophosphoric acids at the 70 % nickel phosphide and minimum in nitric acid and its mixtures with other acids.
Keywords
For citations:
Goikhenberg Yu.N., Polukhin D.S., Zherebtsov D.A., Bodrov E.G. Influence of silicon carbides on the structure and properties of nickel-phosphorus composite coatings. Izvestiya. Ferrous Metallurgy. 2023;66(1):43-49. https://doi.org/10.17073/0368-0797-2023-1-43-49
Introduction
Chrome and nickel plating are the main types of the metal coatings. Currently, the nickel coatings occupy a leading position in the technological process of protecting components [1]. Thus, the composite nickel-phosphorus coatings have a significant wear resistance [2; 3], high corrosion resistance [4 – 7], good adhesion [8] and decorative properties [1]. A promising method of hardening and protecting components of a simple shape is the method of laser cladding of the nickel-based coatings, providing high tribological properties at high temperatures (about 1000 °C) [9; 10]. Methods of obtaining coatings by supersonic electric arc spraying are being studied and improved [11]. According to [12; 13], the most commonly used nickel-phosphorus coatings containing about 10 wt. % of phosphorus in their composition are amorphous after application. A subsequent heat treatment eventually transforms such coatings into a crystalline state which provides the necessary level of properties.
The composite nickel-phosphorus coatings usually have a layered structure, in the surface layer of which there are dispersed particles (silicon carbides, titanium, zirconium, diamond micro-powder [15 – 18]), contributing to the increase in service characteristics. Currently, in the manufacture of critical components used in transportation of oil and gas, the composite nickel-phosphorus coatings with the dispersed silicon carbides are being introduced, which increase the service life of products.
The purpose of this study was to determine the phase composition of the composite nickel-phosphorus coatings with the silicon carbide particles, which provides a high microhardness of more than 1000 HV in combination with a high corrosion resistance in different aggressive media.
Research materials and methodology
We applied a double-layer 60 µm thick nickel-phosphorus coating (Ni-P: 30 µm, Ni-P + silicon carbides: 30 µm), or a single-layer 60 µm thick Ni-P coating to a prepared 300×100 mm, 4 mm thick ground surface made of steel grade 09G2S (EU analog: MnSi5) using the electroless technology with the hypophosphite ions [19; 20]. The single-layer coating composition (% wt.) was as follows: 89.32 – 90.15 Ni; 9.71 – 10.14 P; 0.10 – 0.22 Si; 0.15 – 0.43 Cu. In addition, we also applied the coatings to 3 mm thick ground sheets made of stainless steel grade 08Cr18Ni10Ti (EU analog: X6CrNiTi18-10) in an electroless nickel plating bath. The coatings were subsequently separated by bending for further analysis.
We studied the crystallization of the separated coatings under a continuous heating in a neutral argon atmosphere at rates of 1, 5 and 20 °C/min using a Netzsch STA 449 F1 Jupiter simultaneous thermal analyzer. Then we used the curves obtained by differential scanning calorimetry (DSC) to determine crystallization onset temperatures and to evaluate thermal effects. Under isothermal conditions, the samples of the nickel coatings separated from the substrate were heat-treated according to specified conditions in a LOIP LF-15/11-G1 lab muffle furnace in an oxidizing atmosphere.
We measured the Vickers microhardness at a 100 g load applied to a polished surface of the samples by indenting a diamond indentor on a DuraScan-50 microhardness tester with the ECOS Workflow software. The test procedure was compliant with GOST R ISO 6507-1-2007 (ISO 6507-1:2005). The microhardness measurement error was ±35 HV. We produced flat 20×250×0.06 mm samples of the coating separated from the substrate for tensile testing at a 5 mm/min rate on an Instron electromechanical tensile testing machine with a force of 250 kN. Tensile strength and yield strength measurement error was ±5 MPa, and elongation measurement error was 0.1 %.
The coating resistance to extremely aggressive media was evaluated using the gravimetric method. During the test, the coating was immersed in concentrated acids or their solutions for 24 h at room temperature. Before and after the test, the samples were washed in ethyl alcohol, dried, and weighed with a VLR-200 lab scale (0,25·10–3 g error). The coating weight loss was estimated as a percentage.
The structure of the initial coatings and the coatings after different annealing modes was studied using an Olympus GX-51 inverted microscope. The surface of the prepared sections was etched for 10 s in a mixture of concentrated nitric and acetic acids using the liquid-drop method.
We used a Jeol JSM-7001F Schottky emission scanning electron microscope with an Oxford INCA X-max 80 SDD detector for electron microscopic studies of the structure. The instrument determines the chemical composition of individual structural components and draws distribution patterns of different elements in them.
For X-ray diffraction studies, we used a DRON-4-07 diffractometers (iron anode radiation) and Rigaku Ultima IV (copper anode radiation). We applied the Rietveld method [21] for the qualitative and quantitative phase analysis after optimizing the interference peaks. The accuracy of the quantitative phase analysis was ±5 %. The sizes of the coherent scattering regions (CSRs) were determined by the Williamson–Hall and Halder–Wagner methods [22].
Research results and discussion
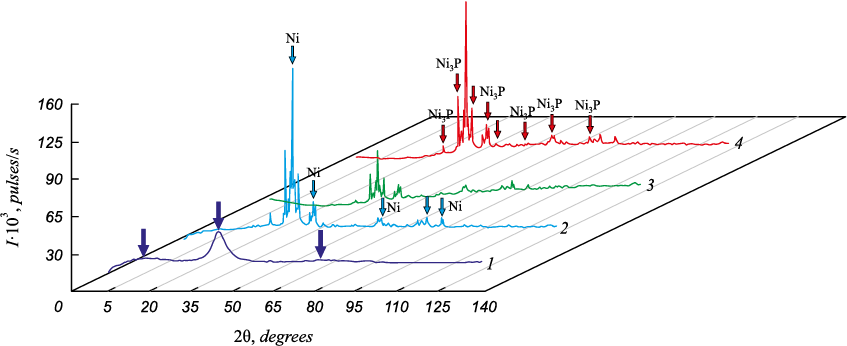
It was found that the coatings containing about 10 wt. % of phosphorus and about 1.0 % of the dispersed silicon carbide particles are in an amorphous state after application to steel substrates. The X-ray diffractograms of such coatings that were not subjected to heat treatment lack any interference peaks. There are only a few halos (marked with arrows) of different intensity in a wide range of 2θ reflection angles (Fig. 1, curve 1).
Fig. 1. X-ray diffractograms of the composite Ni-P coatings with the silicon carbides |
Microhardness of the initial Ni-P coatings is about 400 HV. As 1 % of the silicon carbide particles are added to the solution, it increases to 600 HV. This is also lower than the values of 1000 HV required by the specifications [23]. After application, the strength and plasticity properties of the coatings (refer to Table 1) are low (elongation varies from 0 to 1.5 %).
Heating the nickel-phosphorus coatings leads to crystallization and microhardness increase to the required values (over 1000 HV).
Table 1. Mechanical properties of the Ni-P coatings
| ||||||||||||||||||||
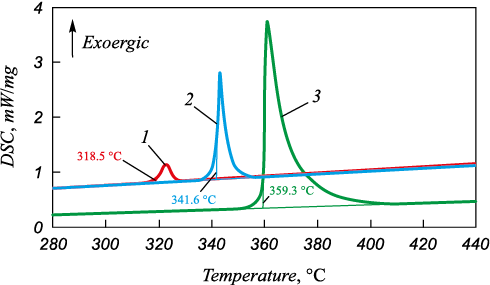
Crystallization onset temperature of the composite Ni-P coating with the silicon carbide particles is above 300 °C during a continuous heating. It largely depends on the heating rate, while a significant exothermic thermal effect changes insignificantly (Fig. 2).
Fig. 2. DSC curves for heating in argon at the following rates: |
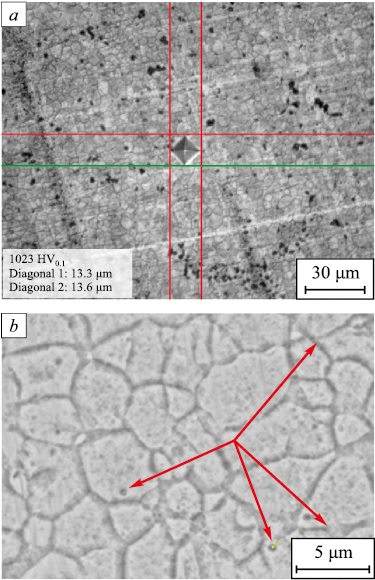
As the samples are heated above the crystallization temperature, the X-ray diffractograms show diffraction peaks (see Fig. 1), indicating the formation of crystalline phases in the coating. As our tests showed, after crystallization, in addition to the SiC and Si5C3 carbides, the coating contains crystallized nickel, and precipitated Ni3P compound. The coating structure is homogeneous and fine-grained. The grain size is 6 – 14 μm, and the silicon carbide particle size is 0.5 – 1.5 μm (Fig. 3, a).
Fig. 3. Microstructure of the Ni-P coating with the silicon carbides |
The crystallization of the Ni-P coating with the silicon carbides develops noticeably under isothermal conditions at a temperature lower than during a continuous heating. For example, it was found that the coatings on a steel substrate contain about 10 % of Ni3P after 24 h of soaking at 280 °C (refer to Table 2). If the temperature or annealing time is increased, the amount of the precipitated crystalline nickel phosphide reaches almost 70 % (refer to Table 2).
Table 2. SCR size, amount of crystalline nickel (СNi )
|
Note that for the coatings containing the dispersed silicon carbide particles, the proportion of these particles did not exceed 1 %. For this reason, we ignored them in the quantitative analysis of the phase composition in the calculation.
With the increase in the content of the nickel phosphide in the coating composition after 15 min of soaking at a temperature of 390 °C, microhardness increases from an initial 600 HV to an average of 976 HV, and to 1057 HV after 120 min of soaking, when 71 % Ni3P is formed (refer to Table 3).
Table 3. Microhardness of the Ni-P coatings
| |||||||||||||||||||||||
When temperature reaches 420 °C, a similar high hardness of the samples is achieved in a shorter time. For all soaking ranges at the specified temperature, hardness is ensured with consistently obtained values of more than 1000 HV.
As the annealing temperature reaches 450 °C, microhardness is at its maximum after 30 min of soaking. Then it decreases due to phase coagulation and phosphorus burnout from the surface which gets a characteristic bluish hue. For a 24 h soaking period at 280 °C, the formed structure creates the same high hardness HVavg = 1016 (1033; 1004; 1023; 1033; 985) HV.
The coatings with a high microhardness after heat treatment have low yield strength and tensile strength. These values are increased by only 12 – 15 MPa. Heat treatment makes the coatings so brittle that their plasticity drops to zero. Such changes in the coating properties during heat treatment are mainly due to the formation of the brittle nickel phosphide compounds with a high microhardness.
The phosphorus microvolume distribution chart helped us to identify the locations of nickel phosphides. Phosphorus in this coating is part of the Ni3P compund. Thus, the locations of phosphorus localization indicate that the nickel phosphides are located both inside the grains and along the grain boundaries. After annealing for 1 h at 420 °C, the nickel phosphide precipitates at all grain boundaries. The nickel phosphides inside the grains and along the grain boundaries are indicated by arrows in the electron microscope image (Fig. 3, b).
The fine structure of the coatings after crystallization annealing in different modes shows that the SCR sizes determined by the Williamson–Hall and Halder–Wagner methods are close. In crystalline nickel, they vary from 10 to 25 nm (refer to Table 2), while in the nickel phosphide they are slightly larger and range from 15 to 30 nm. As listed in Table 2, the lower the annealing temperature and the shorter the soaking time, the smaller the size of the SCR formed.
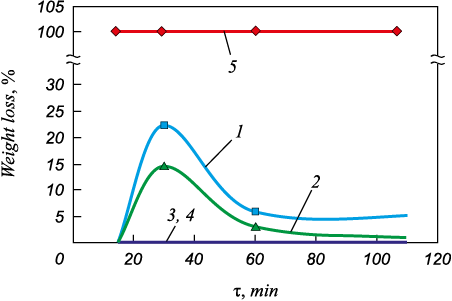
Corrosion resistance of the coatings to the effects of different aggressive media (acids and their solutions) is the most important quality metric along with a high hardness. It was found that the studied Ni-P coating with the silicon carbides has the highest corrosion resistance to acetic and orthophosphoric acids, regardless of heat treatment. After daily tests, the most aggressive medium for such coatings is nitric acid, its mixture with other acids, or even its solution diluted with distilled water. The coatings are completely dissolved in nitric acid and its solutions during daily tests (Fig. 4, curve 5). On the other hand, the max daily weight loss duting soaking in sulfuric acid is less and amounts to 5.3 %. The weight loss in hydrochloric acid is 11.2 %, which is also less than in nitric acid.
Fig. 4. Weight loss (%) vs. heat treatment period at 390 °C |
We also found a relationship between corrosion resistance of the Ni-P coatings with the silicon carbides and their quantitative phase composition. Corrosion resistance is at its maximum after 2 h of soaking at 390 °C (Fig. 4) or 1 h of soaking at 420 °C when about 70 % Ni3P is formed. Its stability is greater than that of pure nickel or its other compounds with phosphorus. The presence of the dispersed silicon carbides decreases the weight loss of the samples compared to the pure Ni-P coating [24]. The introduction of the silicon carbide as a dispersed phase, which creates a barrier effect for the formation of the nickel phosphides, makes it possible to achieve the set goals with the use of a longer treatment at lower temperatures. This also improves main service characteristics of the coating such as a high microhardness and corrosion resistance.
Conclusions
The required 1000 HV of microhardness according to the specifications is achieved in the composite nickel-phosphorus coating with the dispersed silicon carbide particles during a long-term low-temperature annealing with crystallization and the formation of insignificant (10 %) amounts of nickel phosphide.
The revealed dispersed nickel phosphides formed during crystallization and located both inside the grains and along the grain boundaries make a main contribution to the microhardness increase.
A max corrosion resistance of the coatings to different acids combined with a high microhardness is achieved at a high (70 %) nickel phosphide content. Its amount increases with the increase in annealing temperature or time.
With a significant increase in microhardness of the coatings from the initial 600 to the required 1000 HV after crystallization annealing, yield strength and tensile strength increase by only 12 – 15 MPa, and elongation drops to zero due to the formation of brittle nickel phosphide compounds.
Heat treatment of the Ni-P coatings with the silicon carbides forms a homogeneous, fine-grained structure with a grain size of 6 – 14 μm and the SCR size of 10 – 25 nm in nickel, and 15 – 30 nm in the nickel phosphide.
References
1. Moskvitin G.V., Birger E.M., Polyakov A.N., Polyakova G.N. Modern reinforcing coatings of critical parts of mechanisms and tools. Metalloobrabotka. 2015; (2(86)): 22–27. (In Russ.).
2. Alexis J., Etcheverry B., Beguin J.D., Bonino J.P. Structure, morphology and mechanical properties of electrodeposited composite coatings Ni-P/SiC. Materials Chemistry and Physics. 2010; 120: 244–250. https://doi.org/10.1016/j.matchemphys.2009.12.013
3. Aslanyan I.R., Shuster L.Sh. Wear of electrolytic Ni-P coatings during sliding friction. Trudy VIAM. 2015; (3): 52–61. (In Russ.).
4. Drovosekov A.B., Ivanov M.V., Polyakova O.A., Tsupak T.E. Corrosion properties and protective power of Ni-P coatings. Gal’vanotekhnika i obrabotka poverkhnosti. 2011; 19(4): 41‒46. (In Russ.).
5. Ahmadkhaniha D., Eriksson F., Leisner P., Zanella C. Effect of SiC particle size and heat-treatment on microhardness and corrosion resistance of Ni-P electrodeposited coatings. Journal of Alloys and Compounds. 2018; 769: 1080–1087. https://doi.org/10.1016/j.jallcom.2018.08.013
6. Bahramian A., Eyraud M., Vacandio F., Knauth P. Improving the corrosion properties of amorphous Ni-P thin films using different additives. Surface and Coatings Technology. 2018; 345: 40–52. https://doi.org/10.1016/j.surfcoat.2018.03.075
7. Afroukhteh S., Dehghanian C., Emamy M. Preparation of the Ni-P composite coating co-deposited by nano TiC particles and evaluation of its corrosion property. Applied Surface Science. 2012; 28(7): 2597–2601. https://doi.org/10.1016/j.apsusc.2011.10.101
8. Ryabchenkov A.V., Ovsyankin V.V., Zot’ev Yu.A. On the effect of heat treatment of chemically nickel-plated steel on composition and protective properties of nickel-phosphorus coatings. Zashchita metallov. 1969; 5: 638–642. (In Russ.).
9. Makarov A.V., Korobov Yu.S., Soboleva N.N., Khudorozhkova Yu.V., Vopneruk A.A., Balu P., Barbosa M., Malygina I.Y., Burov S.V., Stepchenkov A.K. Wear resistant nickel-based laser clad coatings for high-temperature applications. Letters on Materials. 2019; 9(4): 470–474. https://doi.org/10.22226/2410-3535-2019-4-470-474
10. Makarov A.V., Soboleva N.N., Malygina I.Yu., Osintseva A.L. The formation of NICRBSI–TiC composite coating with increased abrasive wear resistance by gas powder laser cladding. Uprochnyayushchie tekhnologii i pokrytiya. 2013; (11): 38–44. (In Russ.).
11. Kolomeichenko A.V., Logachev V.N., Deev V.B., Dudareva N.Yu. Properties of coatings obtained by supersonic electric arc metallization with aerosol fluxing. Izvestiya. Ferrous Metallurgy. 2022; 65(9): 637–643. (In Russ.). http://doi.org/10.17073/0368-0797-2022-9-637-643
12. Pillai A.M., Rajendra A., Sharma A.K. Electrodeposited nickel-phosphorous (Ni – P) alloy coating: An in-depth study of its preparation, properties, and structural transitions. Journal of Coatings Technology and Research. 2012; 9(6):785–797. https://doi.org/10.1007/s11998-012-9411-0
13. Buchtík M., Krystýnová M., Másilko J., Wasserbauer J. The effect of heat treatment on properties of Ni-P coatings deposited on AZ91 magnesium alloy. Coatings. 2019; 9(7): 461. https://doi.org/10.3390/coatings9070461
14. Gorbunova K.M., Nikiforova A.A. Physico-Chemical Basics of Chemical Nickel Plating. Moscow: USSR Academy of Sciences; 1960: 7–194. (In Russ.).
15. Sotskaya N.V., Dobrozrakova A.D., Aristov I.V., Ryabinina E.I. Features of the formation of composite coatings with inclusion of diamond micro-powder by chemical nickel plating. Teoriya i praktika sorbtsionnykh protsessov. 1998; (3): 114–120. (In Russ.).
16. Mainier F., Fonseca M.C., Tavares S., Pardal J. Quality of electroless Ni-P coatings applied in oil production equipment with salinity. Journal of Materials Science and Chemical Engineering. 2013; 1(06): 1–8. https://doi.org/10.4236/msce.2013.16001
17. Joseph A., Narayanasamy M., Kirybasankar B., Angaiah S. Development of MoS2 nanosheets embedded nickel composite coating and its mechanical properties. ES Materials & Manufacturing. 2018; 2: 2–8. https://www.doi.org/10.30919/esmm5f152
18. Osama F., Radwan A.B., Sliem M.H., Abdullah B.M., Hasan A., Shakoor R.A. Investigating the properties of electrodeposited of Ni-P-ZrC nanocomposite coatings. ACS Omega. 2021; 6: 33310–33324. https://doi.org/10.1021/acsomega.1c03117
19. Gorbunova K.M., Ivanov M.V. Chemical Methods of Metal Deposition (Chemical Nickel Plating and Cobalting): Handbook. Moscow: Metallurgiya; 1987: 365–401. (In Russ.).
20. Gamburg Yu.D. Chemical Nickel Plating (Obtaining Nickel–Phosphorus Coatings by Electrocatalytic Reduction with Hypophosphite). Moscow: RAS; 2020: 82. (In Russ.).
21. Krzhizhanovskaya M.G., Firsova V.A., Bubnova R.S. Application of the Rietveld Method for Solving Problems of Powder Diffractometry. Manual. St. Petersburg University; 2016: 67. (In Russ.).
22. Izumi F., Ikeda T. Implementation of the Williamson–Hall and Halder–Wagner methods into RIETAN-FP. Annual Report of Advanced Ceramics Research Center. 2014; 3: 33–38.
23. Goikhenberg Yu.N., Polukhin D.S Structure, properties and quality of composite nickel-phosphorus coating applied to steel substrates of various composition. Chernye metally. 2022; (4): 46–49. (In Russ.). http://doi.org/10.17580/chm.2022.04.08
24. Polukhin D.S., Goikhenberg Yu.N., Bodrov E.G. Corrosion resistance of composite nickel-phosphorus coating in various aggressive media. Voprosy materialovedeniya. 2022; (3(111)): 98–108. (In Russ.). http://doi.org/10.22349/1994-6716-2022-111-3-98-108
About the Authors
Yu. N. GoikhenbergRussian Federation
Yurii N. Goikhenberg, Dr. Sci. (Eng.), Senior Researcher, Prof. of the Chair of Materials Science and Physical Chemistry of Materials
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
D. S. Polukhin
Russian Federation
Dmitrii S. Polukhin, Executive Director
8 Eniseiskaya Str., Chelyabinsk 455030, Russian Federation
D. A. Zherebtsov
Russian Federation
Dmitrii A. Zherebtsov, Dr. Sci. (Chem.), Senior Researcher of the Chair of Materials Science and Physical Chemistry of Materials
76 Lenina Ave., Chelyabinsk 454080, Russian Federation
E. G. Bodrov
Russian Federation
Evgenii G. Bodrov, Director
8 Eniseiskaya Str., Chelyabinsk 455030, Russian Federation
Review
For citations:
Goikhenberg Yu.N., Polukhin D.S., Zherebtsov D.A., Bodrov E.G. Influence of silicon carbides on the structure and properties of nickel-phosphorus composite coatings. Izvestiya. Ferrous Metallurgy. 2023;66(1):43-49. https://doi.org/10.17073/0368-0797-2023-1-43-49