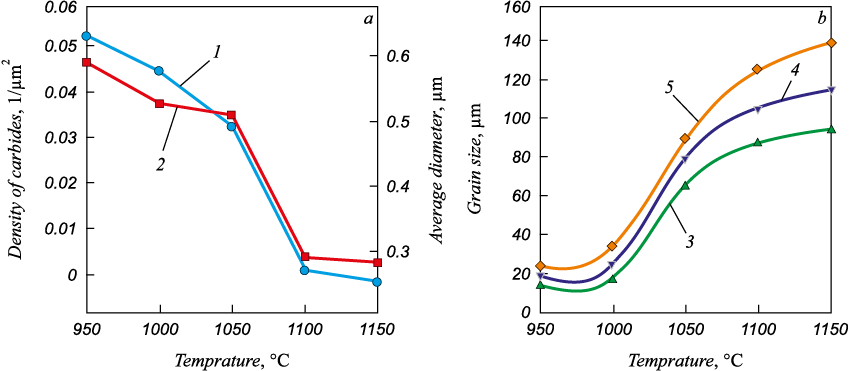Scroll to:
Corrosion-resistant steels based on Fe – ~13 % Cr: Heat treatment, corrosion- and wear resistance. Review
https://doi.org/10.17073/0368-0797-2023-1-8-26
Abstract
Martensitic stainless steels with 13 % Cr are widely used in many industries due to their high level of mechanical properties and acceptable corrosion resistance. The paper consolidates information on the guaranteed level of properties and heat treatment conditions required for its implementation. The properties after treatments proposed by researchers are compared with those known for industrial metal. The dependences of the hardness of 13Cr type hardened steels with 0.20 – 0.50 % C on the austenitization temperature and the accompanying changes in structure have been analyzed. The temperatures providing maximum hardening and the temperatures at which the steel ceases to harden have been revealed. The effect of the duration of austenitization, heating and cooling rates on the properties of steels has been considered. The mechanical properties and corrosion resistance after quenching, quenching and tempering in relation to structural-phase states of steels are considered. It is discussed in detail how the type of secondary phases during tempering, their amount, and distribution affect the corrosion resistance of steels with 13 % Cr. It increases with increasing heating temperature during austenitization and decreases with increasing tempering temperature due to the precipitation of Cr23C6 carbides and depletion of the matrix in chromium to the concentrations below 12 %. The tempering temperature of 500 – 550 °С is recognized as the worst: due to intensive precipitation of carbides the steel is not passive, and the corrosion rate is maximum. Quenching with low tempering is recommended for 20Cr13 steels (to combine high strength, good corrosion resistance and satisfactory plasticity), or, more often, quenching with high tempering is recommended at ~(650 – 700) °С (good plasticity, satisfactory corrosion resistance). For steels of 40Cr13 type the temperature of ~700 °С is not recommended because of the increased concentration of carbides and insufficient corrosion resistance. Examples of increasing the wear resistance properties of 40Cr13 steels due to surface treatments, from nitriding to laser and plasma surface quenching, are presented.
Keywords
For citations:
Kostina M.V., Rigina L.G., Kostina V.S., Kudryashov A.E., Fedortsov R.S. Corrosion-resistant steels based on Fe – ~13 % Cr: Heat treatment, corrosion- and wear resistance. Review. Izvestiya. Ferrous Metallurgy. 2023;66(1):8-26. https://doi.org/10.17073/0368-0797-2023-1-8-26
Introduction
Medium-carbon high-strength martensitic steels with 0.20 – 0.40 % C and 12 – 14 % Cr are a widely demanded constructional material, which is the most inexpensive among corrosion-resistant steels. They are used in the manufacture of loaded parts, friction pairs and metal seals, pressure vessels, hydraulic units, casings for the oil and gas industry, and steam turbine blades. Although they are not a new material, there are many publications dedicated to them in the scientific literature. These works are aimed at:
– modifying the surface of (20–40)Cr13 steels to increase their strength and wear resistance properties, studying their corrosion resistance;
– forming the structure and phase composition of similar steels, providing high strength while maintaining the process ductility and ensuring corrosion resistance due to variations in the chemical composition and heat treatment modes.
In this review article:
– information on the structure and guaranteed level of properties currently achievable in industrial steels with 0.20 – 0.40 % C and 12 – 14 % Cr is provided;
– the structure and mechanical properties of steels of this type, obtained as a result of modern studies of the effect of different variants for traditional heat treatment of such steels – martensite quenching and different types of tempering (annealing) – are considered;
– information on the results of studies of corrosion resistance of these steels is given.
Properties of industrial steels with ≤0.20 – 0.40 % С and 12 – 14 % Сr
When heated above 800 °C, austenite appears in steels with 13 % Cr. The carbon concentration increase contributes to the expansion of the γ-region1 [1]. Dissolution of carbide phase particles (primary carbides) occurs during high-temperature annealing. Cooling from the austenitic region fixes the martensitic structure in the steel. Depending on the quenching heating temperature and steel composition, some carbide, ferrite or residual austenite particles may be present in it. During the tempering process, depending on the temperature and duration of the process, there may be a return, polygonization, recrystallization, nucleation of secondary dispersed carbides in martensite, their growth and coagulation. In this way, it is possible to obtain a structure consisting of tempered martensite with carbides, or to bring the process to the decomposition of martensite into a ferrite-nitride mixture.
Table 1 provides standard grade chemical compositions of common industrial steel grades with <0.20 – 0.40 % C and 12 – 14 % Cr. In Russia these are steel grades 20Cr13, 30Cr13 and 40Cr13, differing in carbon content only. According to standard GOST RF 5632-2014, they do not contain other metallic alloying elements except chromium (and up to 0.8 % Mn, see Table 1). Such steels are also known to be supplied with up to 0.6 % Ni, up to 0.2 % Ti, and up to 0.3 % Cu2. Steel AISI 420 is an analogue of all the mentioned Cr13 grades with 0.2 – 0.4 % С, because its carbon content is limited to the lower limit of 0.15 %, but the upper limit is not specified3 (see Table 1).
Table 1. Chemical composition, % (wt.), of Russian and foreign steel grades
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Using reference resources 2, 3, 4, 5, 6, 7, 8, the authors summarized the information on industrial steels of Cr13 type (13Cr are foreign grades):
– critical points, treatment modes and structure (Table 2);
– impact of the tempering temperature after quenching on their mechanical properties (Table 3);
Table 2. Process parameters and structure of steels 20Cr13, 30Cr13, 40Cr13 (according to 2, 4, 5 )
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 3. Mechanical properties at 20 °С of 20Cr13 and 40Cr13 6 and AISI 420 7
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
– mechanical properties of semiproducts from these steels, giving the idea of their guaranteed level of properties, which modern researchers try to surpass (Table 4).
Table 3 shows that high annealing (tempering) at 700 °C causes increased ductility and impact strength, because at this temperature martensite in steel is converted to ferrite and carbides (see Table 2). The yield strength of rods and forgings varies depending on the section and carbon concentration from 440 to 635 MPa, the tensile strength from 510 to 830 MPa, and the ductility from 12 to 16 %. After quenching and low tempering at 200 to 300 °C, these steels have high strength and low ductility (see Table 3). Therefore, for Russian industrial semiproducts after such treatment only hardness values are given (see Table 4), and for semiproducts made of AISI 420 steel the data of tensile tests are also given. Table 4 shows that for semiproducts from Cr13 type steel the main type of heat treatment is quenching from 1000 – 1050 °С and tempering, mainly high, at temperatures in the range of 600 – 770 °С.
Table 4. Mechanical properties of semiproducts from 4Cr13 type steels according
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Studies of the impact of quenching
and tempering (aging) processes on the structure
and properties of Cr13 type steels
At the end of this section summary Table 5 is presented with the chemical composition of all steels considered here.
Hardness measurements are most often used to evaluate the mechanical properties of Cr13 type steels, since it correlates with strength. Few tensile and impact bending test results given in the literature are collected in separate summary Table 6 at the end of this section.
Table 5. Chemical composition of 13Cr steels under study
|
Heating temperature for quenching (austenitizing)
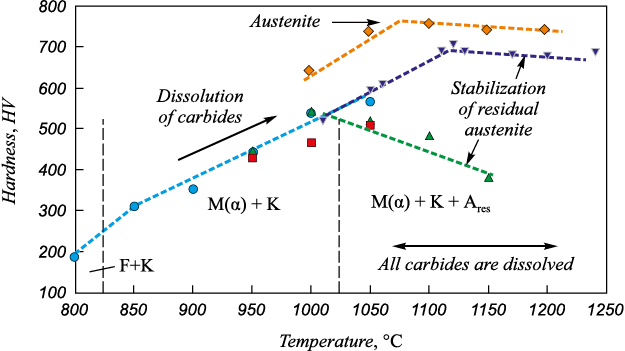
It is known that hardening during martensite quenching of steels is caused by several factors and primarily high dislocation density and presence of carbon in the solid solution. The results of studies [2 – 5] on the effect of austenitizing temperatures of Cr13 type steels with 0.14 – 0.45 % C before quenching on their hardness and phase composition are presented in Figure 1. After holding at 800 °C [2] or rolling at 850 °C [4] and quenching in oil, the steel has a structure consisting of ferrite and finely dispersed Cr23C6 carbides (F + C) and is characterized by minimum hardness. Increasing the heating temperature for quenching to t ≥ 850 °C causes partial dissolution of carbides and fixation of martensitic structure (M(α)) in the steel during quenching [2]. As the austenitizing temperature increases due to the intensification of carbide dissolution, the hardness of martensite-quenched steel increases. This is due to a significant increase in the degree of tetragonality (с/а) of the martensite crystal lattice, described by dependence [6]
| с/а = 0.45[C] + 1.00. | (1) |
Fig. 1. Effect of austenitization temperature9 before quenching on hardness10 |
Herewith, in Cr13 steels parameter с/а with increasing carbon content increases 2.5 times more intensively than in similar unalloyed steels [2].
The maximum values of HV 540 – 570 for 20Cr13 type steels are achieved after quenching from the temperatures of 1000 – 1050 °С [2 – 4]. For steel with 0.45 % C, the maximum HV level of 696 – 710 is achieved after quenching from 1110 – 1130 °C [5] (see Figure 1). Large-needle martensite is noted in the samples quenched from 1000 °C [2].
When comparing the X-ray diffraction spectra of annealed (α-Fe) and austenitized and martensite-quenched (M(α)) samples, expansion and shifting of peaks are clearly visible, which is due to the stress state of the martensitic lattice due to its saturation with carbon [3]. Peak shifting increases with increasing quenching temperature, which indicates greater dissociation of chromium carbides with temperature and increased saturation of martensite with carbon.
The noticeable effect of hardness reduction after reaching its maximum during further increase of the heating temperature over 1000 °C, recorded for 20Cr13 steel with 0.08 % N (420U6) [4], 45Cr13 and 50Cr13 steels during heating at 1100 °C and above [5, 7] is explained by the following:
– in the structure of these steels due to the intensification of carbides and carbonitrides dissolution the concentration of austenite-forming elements (carbon [5, 6], carbon and nitrogen [4]) is achieved and increases, contributing to the formation of residual austenite and increasing its quantity after quenching (Fig. 1);
– growth of the austenite grain [7].
It is noteworthy that in the 20Cr13 steel, in the absence of nitrogen in its composition, stabilization of austenite after holding at 1050 °C did not occur [2, 3] in contrast to the 20Cr13 steel with 0.08 % N [4] (Fig. 1). It should be noted that in the 50Cr13 steel, which is on the modified Schaeffler–Delong diagram in the martensite-austenite region near the boundary with the austenite region, the amount of austenite after austenitization at temperatures in the range of 1000 – 1200 °C and quenching increases from 97.5 to 100 % [7].
Grain-boundary carbides not dissolved during thermal soaking inhibit grain growth during heating. Increasing the austenitizing temperature of the 20Cr13 steel with 0.08 % N from 950 to 1100 °C (holding during 30 min) leads to an order decrease in carbide density from ~0.053 to ~0.004 1/µm2, and their average diameter decrease from 0.57 to 0.26 μm (Fig. 2, a) [4]. Further increase in the annealing temperature to 1150 °C no longer contributed to significant changes in the particle density and size. Increasing the austenitizing temperature from 950 to 1000 °C did not cause the grain growth during holding for 30 and 60 min at those temperatures, and the grain size remained equal to 15 – 18 μm. Increasing the heating temperatures above 1000 °C led to significant grain growth (Fig. 2, b). Obviously, decrease in the carbide density and increase in the grain size also contribute to the decrease in hardness of this steel quenched from temperatures above 1000 °C.
Fig. 2. Effect of austenitization temperature of 20Cr13 steel with 0.08 % N |
Only weak grain growth from 10 to 20 µm was observed for the 45Cr13 steel with higher carbon content [5] in the heating tempering range for quenching of 1000 – 1120 °C; austenitization at 1170 and 1240 °C resulted in the grain growth to 47 and 65 µm respectively. For steel X46Cr13 (1.4034) it was noted [9] that austenitizing at temperatures above 1100 °C causes complete dissolution of carbides in X46Cr13 and optimum distribution of chromium and carbon in the mixed crystal. The elimination of the blocking effect of carbides and the higher diffusion rate lead to significant grain enlargement. Decreasing the austenitizing temperature below 1100 °С leaves mixed chromium and iron carbides in the structure, which reduce hardness and corrosion resistance.
Duration of heating during austenitization
(during heating for quenching)
The effect of duration of annealing (~950 – 1200 °C, 30, 60 and 120 min) of the 20Cr13 steel with 0.08 % N on the structure and hardness has been studied [4]. It is shown that the longer the holding time at a given temperature, the coarser the grain size, and this effect is more significant the higher the heating temperature (Fig. 2, b). At low temperatures (960 and 1000 °C), the holding time had little effect and the grain growth from the 15 – 20 μm level was practically not registered. At 1200 °С such holding led to the grain growth up to 87 – 142 μm. With all holding times, the hardness maximum was observed when the austenitization temperature increased to 1000 °С, and then it decreased with increasing temperature. The longer the holding time in the range of 1050 – 1150 °C, the more residual austenite was in the steel and the lower the hardness was achieved during subsequent quenching. Treatment at 1000 °C for 30 min was chosen as optimal, as it provided maximum hardness while maintaining a relatively fine grain size.
Thus, the maximum effective temperature of austenitization before quenching, which provides high hardness, is 1000 – 1020 °C for steel type 20Cr13 and 1100 – 1120 °C for steel type 45Cr13.
Heating rate during austenitization
and cooling rate during quenching
When quenching carbon-containing steels, martensitic transformation occurs in a shear manner. However, this does not exclude the possibility of diffusive redistribution of carbon in austenite during cooling to the temperature of the beginning of martensitic transformation (Mn ) and further in the formed martensite when cooling from Mn to the room temperature [6].
The study [10] conducted on steel with 0.45 % C and 13Cr (45Cr13) showed that the temperature required to achieve complete dissolution of Me23С6 carbides in the austenitic phase increases with the increasing heating rate from 0.05 to 10 K/s, changing from 1353 to 1448 K (1080 – 1175 °C). For a given heating rate and holding time (60 s), the amount of carbide in the quenched microstructure of this steel decreases with increasing temperature. Carbide precipitation was found during quenching from 1393 K (1120 °C) and slower cooling rates than 20 K/s. For these cooling rates, the amount of carbide precipitation increased with the decreasing cooling rate. With continuous cooling at any quenching rate from 1333 K (1060 °C) no significant carbide precipitation is observed. After annealing at optimum temperatures, starting from the cooling rate of 1 °С/s, the hardness of martensite microstructures is very close to the maximum. The hardness obtained by quenching from their respective optimum temperatures reaches the values between 700 and 710 HV5 when cooling at 1 °C/s. For X45Cr13 steel heated to 1120 °C, the percentage of the carbide area in the final microstructure after quenching at a cooling rate of 1 °C/s is 3.2 %, whereas when quenched from 1060 °C at a cooling rate of more than 25 °C/s it is 6 % [10].
High-rate heating (50 °С/s) by the method of current transmission through the specimen was carried out on steel 20Cr13 specimens (length of 100 mm, diameter of 10 mm), after which they were quenched in oil [2]. The obtained properties were compared with the results of metal heated in the furnace and similarly quenched metal. The maximum value of the tensile strength of 1530 MPa when heated in the furnace was achieved after quenching from 950 °C, and the relative elongation did not exceed 4.7 %. During high-rate heating the same strength was obtained after quenching from a temperature of 1020 °С, and the relative elongation in this case did not exceed 6.5 %. After a series of experiments in [2] it was concluded that high-rate heating leads to a shift of hardening curves by 40 – 60 °С up the temperature scale compared to the curves obtained during furnace heating.
The effect of different cooling rates (from 3 to 100 K/s) during hot forging of X46Cr13 steel on the hardness, strength and ductility of steel after quenching (1100 °C, 300 s) and after additional tempering (1100 °C, 300 s) was studied [11]. This factor was shown to have no effect on hardness: it is at a level of about 700 HV10 after quenching and 580 HV10 after tempering. During the study of the effect on strength and ductility, the steel sheets were cooled to the room temperature at a rate of 3 to 140 K/s, caused by different surface pressures in the tool and outside the cooling medium. No significant effect on the tensile strength was found, whereas the relative tensile elongation could decrease from 11 to 6 % with the increasing cooling rate. The best properties (strength of 1800 MPa and relative elongation of 11 %) were obtained after a low surface pressure of 1 MPa and a cooling rate of 30 K/s.
Effect of tempering modes after quenching
from different temperatures
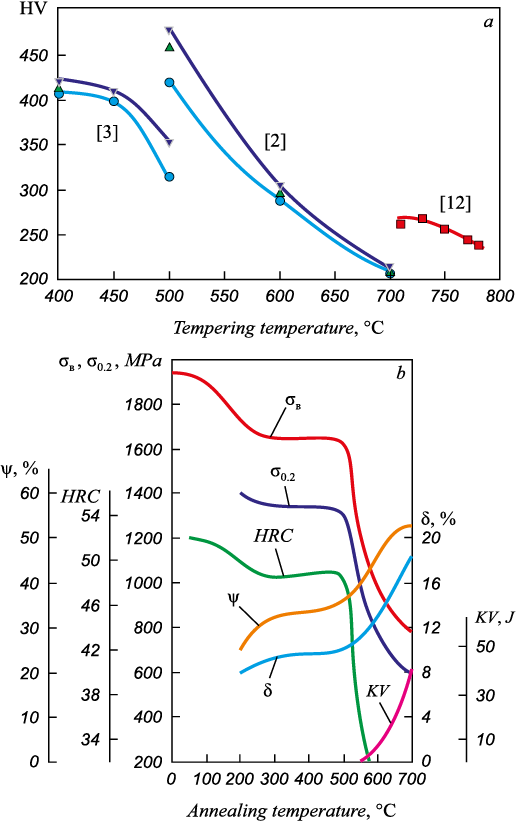
Tempering of quenched laboratory steels 20Cr13 [2], AISI 420 with 0.17 % C [3] and <0.20 % C [12] causes their hardness reduction especially significant in the temperature range from 400 to 780 °С (Fig. 3, a). In the tempering temperature range up to ~600 °C higher hardness values are inherent in steels quenched from higher temperatures, which have higher supersaturation of austenite with carbon during quenching (this shows a significant difference in hardness values for the same tempering temperature obtained in different studies). The results of the study of properties after quenching and tempering over the widest temperature range are given for the X30Cr13 steel (1.4028) with a grade content of 0.26 – 0.35 % C and up to 1 % Si (Fig. 3, b)11.
Fig. 3. Effect of annealing temperature: |
The annealing temperature range of 710 – 780 °С was studied in [12] due to the fact that the 13Cr steel casings are used in the condition after quenching and tempering at 680 – 780 °С (API-5CT). After quenching from 975 °C, the steel was characterized by the presence of lath martensite and hardness of 525 HV. Holding of such martensite for 20 min at 710, 730, 750, 770, 780 °С showed that tempering at ~(710 – 730) °С leads to martensite enlargement. It becomes equiaxial, and in its structure there are Cr23C6 carbides in the form of spheres/rods and needle Cr7C3 carbides (~100 nm). Tempering at 770 °C causes dissolution of Cr7C3 carbides and enlargement of spherical Cr23C6 carbides, and recrystallization occurs. Hardness at such high tempering decreases (Fig. 3, a).
Hardness of the X30Cr13 steel weakly decreases in the temperature range up to 300 °C, then a plateau is observed up to 500 °C, after which, in the range of 500 – 600 °C, there is a sharp decrease in hardness (Fig. 3, b). The strength properties change in a similar way, including the ultimate strength decreasing from 1600 to 900 MPa for anneals between 500 and 700 °C. The ductility and impact strength change mirror-like, and when annealed at temperatures above 500 °C they increase significantly. The manufacturer recommends 11 the following temperatures for this steel: 900 – 1100 °C for hot deformation, 745 – 825 °C for annealing with cooling in the air, 950 – 1050 °C for quenching in oil or air, and 625 – 675 °C for annealing (after quenching from 850 °C).
The effect of isothermal holding of steel quenched from 975 °C at 750 °C for 5 – 60 min on the carbide formation processes was studied [12]. After isothermal treatment for 5 min, Cr23C6 carbides were formed mainly at the grain and lath boundaries, and Cr7C3 carbides were formed inside the laths. Further increase in the time of isothermal annealing led to dissolution of Cr7C3 carbides and enlargement of Cr23C6 carbides. Accordingly, after holding for 5 and 15 min, the return processes were observed, and after longer holding recrystallization and grain growth processes took place. The return and recrystallization during tempering reduce the hardness of steels up to 250 HV. Minimum hardness at 750 °C is achieved during 15 min holding, at which time it decreases from 550 to 275 HV. Further heating at 750 °C (up to 60 min) does not lead to changes in hardness. In this case, the average particle size increases from ~45 to ~130 nm, and their density decreases compared to the maximum one by a factor of 3. The density of the particles is maximum after holding for 5 min; and during this time about 50 % of the total amount of the carbide phase is precipitated for 60 min, estimated by the “area fraction, %” parameter.
In [11] the heating temperatures for quenching of the X20Cr13 steel varied from 950 to 1150 °С and the tempering temperatures were 225, 375 and 525 °С. The holding times during such treatments were 240 and 480 s. The strength of the steel in this case ranged from 1310 to 1660 MPa, and the ductility varied from 3.5 to 7.5 %. The best combination of these characteristics, 1515 MPa strength and 7.5 % elongation, was achieved after quenching from 1050 °C (240 s) and tempering at 375 °C (420 s).
In this section only the effect of tempering on the structure and mechanical properties of steels is considered; below, in a separate section, attention is paid to the effect of this treatment on the corrosion resistance of steels with 13 % Cr.
Use of complex heat treatments:
repeated austenitization, double annealing, cooldown
The effect of double annealing on the structure, hardness, strength and impact strength of AISI 410 steel was studied [13]. In the initial state the steel had a structure consisting of ferrite and chromium-rich carbides Me23C6 after annealing at 750 °C for 2 h followed by slow cooling inside the furnace to a temperature of 25 °C for 20 h in order to obtain maximum softness for molding [13, 14]. Such samples were heated in the range of 900 and 1100 °C (30 min) and quenched in oil, followed by double annealing at temperatures between 200 and 700 °C (steel was cooled after annealing and then annealed again at the same temperature). The purpose of repeated annealing was to promote the transformation of residual austenite into martensite, since, according to [15], residual austenite is almost completely transformed as a result of double tempering at high temperature.
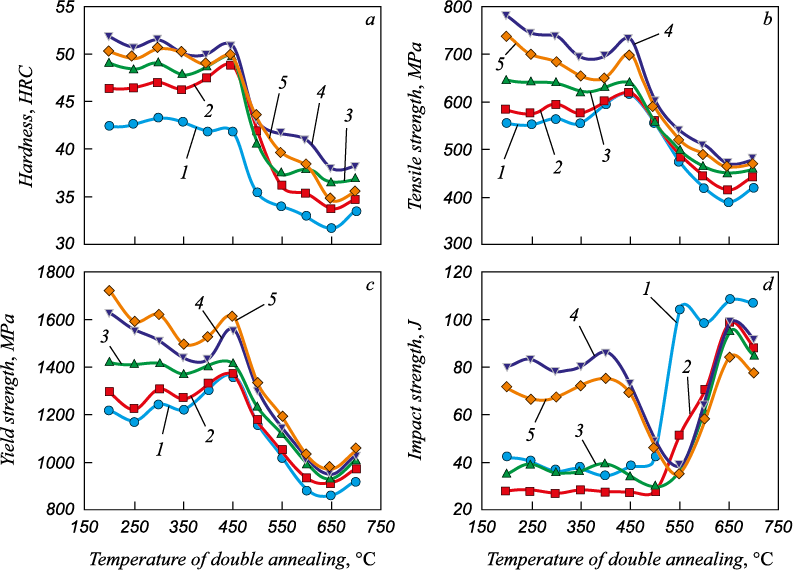
It was shown [13] that chromium carbides Me23C6 dissolve in the temperature range from 950 °C. Varying the tempering temperature of steel samples austenitized at 900 °C does not effectively change the microstructure or cause hardening (Fig. 4, a), as Me23C6 carbides are not precipitated, martensite and ferrite become softer and ductility increases. The structure after this treatment is ferrite in a matrix of lath-tempered martensite with Me23C6 chromium carbide particles (primary and small particles of secondary). The highest values of hardness as well as the yield strength and tensile strength are achieved after quenching from higher ТА = 1050 °С and tempering at 200 °C (Fig. 4, a – c).
Fig. 4. Effect of double annealing temperature on hardness (a), yield strength (b), |
The microstructure after tempering at 200 – 650 °C consists of ferrite islands and small spheroidal particles of secondary chromium carbide Me23C6 in a matrix of coarse-grained lath-tempered martensite. Tempering at t ≥ 550 °C leads to an increase in the number of precipitations along grain boundaries. A satisfactory combination of hardness, strength and impact energy is achieved by double tempering of steel at 200 and 450 °C after quenching from 1050 °C (Fig. 4, Table 6) [22]. In general, double tempering did not result in a significant change in mechanical properties for any of the tested specimens; the microstructure after it still contained a significant amount of residual austenite. During conventional austenitizing treatment, carbide dissolution and grain size growth intensified with increasing austenitizing temperature, while double tempering treatment promoted carbide formation with a slight increase in the grain size. For comparison, in the 40Cr13 type steel (with 0.38 % C and 0.3 % V, i.e., in which the number of carbide particles must be much larger) the precipitations in the samples after single tempering at 300, 500 and 650 °C are nanosized ε-Me3C carbides, chromium-rich nanosized Me23C6 carbides and micron or submicron Me23C6 carbides, respectively [16].
The effect of treatment with double quenching and double annealing (710 °C + 680 °C) on the microstructure, hardness, and mechanical properties of 13Cr hot-rolled steel with 0.2 % C was studied [17]. Austenitizing followed by quenching (duration of 3 h 15 min) was carried out according to the following modes: 980 °C, quenching + 1040 °C, quenching; 1040 °C, quenching + 980 °C, quenching. Cooling during quenching and after tempering was carried out in oil. Both in the case of single quenching at 980 °C and double quenching (1040 °C + 980 °C), there was no delta-ferrite in the tempered martensite microstructure. After single heat treatment, the structure contained carbides along the grain boundaries, and very fine distribution of ferrite was observed. During single quenching, continuous carbide chains along the grain boundaries of the former austenite contributed to the reduction of the impact strength, and its values did not meet the specification requirements. When this steel with the initial martensite microstructure obtained during the first quench from 1040 °C was subjected to secondary austenitizing at 980 °C, recrystallization of the grain structure from the defective matrix of martensitic laths obtained during the first quench occurred. The modified heat treatment with double quenching at 1040 °C + 980 °C provided a finer grain size along with a higher degree of carbon dissolution in the austenitic matrix. During tempering, very fine carbides (having a much smaller size compared to the single heat treatment process) formed in small numbers at low-angle and high-angle boundaries. This resulted in the increased strength and impact strength after tempering, compared to single quenching from 980 °C (Table 2).
In [18] the effect of conventional heat treatment and cryogenic treatment on the mechanical properties of AISI 420 steel was compared. Cryogenic treatment was carried out by a gradual decrease in temperature to avoid the thermal shock: –20 °C, 4 h; –70 °C, 5 h; –196 °C, 24 h. Subsequent heating occurred in the reverse sequence. In the initial state (annealing at 850 °C and cooling with a furnace) the steel had a ferrite-carbide structure with low mechanical properties. Quenching to martensite from 1000 °С followed by tempering at 200 °С provided a martensite structure with residual austenite and undissolved dispersed carbides, and a combination of strength of 989 MPa with ductility of 15 %. Increasing the tempering temperature to 500 °C resulted in coarsening of Me7C3 carbides and partial transformation to Me23C6 carbides, some reduction in strength and increase in ductility. Conducting a stepwise cryogenic treatment before tempering at 500 °С increased the strength properties to 933 MPa and the relative elongation to 40 % (Table 6) due to the precipitation of finely dispersed carbides. The combination of strength and plastic properties thus obtained for this steel is a good result, but the disadvantage of such treatment is the complexity of cryogenic treatment using long periods of holding in a refrigerator, dry ice and liquid nitrogen and subsequent heating in the reverse sequence.
Suggested heat treatment options and mechanical properties
Data on the chemical composition of 13Cr type steels discussed above are given in Table 5. The mechanical properties obtained by researchers for 13Cr steels when varying both conventional quenching and tempering modes and dual heat treatments are given in Table 6. Comparison of the properties of treatments No. 1 – 21 from Table 6 with the properties of industrial steels of the 13Cr group (Table 3) shows that treatments No. 1 and 2 for 20Cr13 type steels and treatment No. 21 for the 40Cr13 steel achieved a higher level of strength than that specified in the known reference materials for these steels. After treatments No. 4 and 5, the 20Cr13 type steel had a level of strength close to that of this steel after tempering at 700 °C in Table 3, but a higher ductility was achieved in this case. The results of treatments No. 16 – 20 are new, and in the reference literature there is no such data for the 30Cr13 steel.
The publications dedicated to the study of corrosion resistance and the possibilities to increase the wear resistance of steels of the Cr13 group are considered below.
Table 6. Mechanical properties of 13Cr steels after various heat treatments
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Studies of wear resistance of steels with 13 % Cr
In the Russian scientific segment, a number of publications have been found that consider the prospect of increasing the wear resistance of 40Cr13 steel due to surface treatments. In addition, a significant place is given to the surface layer saturation with nitrogen during the following treatments:
– nitrocementation [19];
– ion-plasma nitriding [20, 21], including thermal-cycle [20];
– nitriding combined with heat treatment [22].
It is demonstrated that diffusion layers on the cutting surfaces of 40Cr13 steel, saturated with large amounts of carbonitrides during nitrocementation, provide high cutting ability, self-sharpening and wear resistance [19]. Ion-plasma thermal-cycle nitriding made it possible to obtain hardened wear-resistant surfaces, which have a complex of specific physical, mechanical and operational properties [20]. It has been established that during high-frequency nitriding of 40Cr13 steel in inductively coupled plasma of the argon, hydrogen and nitrogen mixture a three-layer structure is formed in the near-surface layer. Its wear rate is the lower the higher the amplitude of the displacement potential [21]. A study of the wear mechanism of ion-modified nitrogen in 40Cr13 steel subjected to various modes of pretreatment has shown that the nitrated layer is an α-Fe matrix phase with chromium nitrides CrN. In the process of friction of nitrogen-modified 40Cr13 steel, accelerated wear of the nitrided layer is registered as its thickness decreases to a certain critical value. As the hardness of the substrate increases, the critical thickness of the nitrided layer decreases from 11 – 12 to 9 – 10 μm [22].
The possibilities of hardening the 40Cr13 steel by surface laser and plasma quenching have been studied [23, 24]. The possibility of effective surface hardening of products using laser heating is also considered. The influence of arising thermal stresses on the temperature interval of austenitic transformation is taken into account, and the dependences of hardness on density, power and treatment rate are analyzed. The work showed that high hardness is achieved when heating to a temperature of 150 – 200 K below the melting temperature [23]. The technology of plasma surface hardening of products made of high-alloy corrosion-resistant steel 40Cr13 allows obtaining a hardened martensitic layer more than 4 mm deep on its surface [24]. The feature of the technology is the microhardness values evenly distributed over the section, the absence of changes in the geometric shape and structure of the 40Cr13 steel part core. In the hardening zone from the solid phase a spectrum of structures is observed – from the martensitic type structure on the boundary with the melting zone with the transition to the martensitic type structure with carbides precipitation (both in the grain body and on the grain boundaries). In the transition zone (thermal impact zone) the structure has the form of a ferrite-carbide mixture of sorbitic type of different dispersion. Such a distribution of microstructures in zones is characteristic of the traditional hardening of 40Cr13 steel products for maximum hardness with preservation of corrosion resistance properties.
Complex treatment of the 40Cr13 steel consisting of heat and mechanical treatments, high-vacuum annealing and diffusion siliconizing has been proposed [25]. It provides the possibility of hardening to a depth of 4.2 mm. Tests of fracture and wear resistance, evaluation of the hardness and microgeometry of the surface layer of samples showed that this treatment can increase the durability of parts.
The use of the 40Cr13 steel as a coating on steel 45 to increase the wear resistance of the material is of interest [26]. Gas-thermal coating of 40Cr13 wire steel was applied to steel 45 plates by high-speed metallization. Additionally, the coating was treated with nitrogen ions. Ion-beam treatment increases the microhardness of coatings to the values of 1000 – 1450 HV0.025 and their wear resistance under friction in the I-20 lubricant medium by 1.7 times. Based on the results obtained, the temperature mode of ion-beam nitriding with the highest tribotechnical properties has been selected.
Studies of corrosion resistance of steels with 13 % Cr
It is known that heat treatment is an important factor influencing the tendency of alloys to corrosion. Stainless steels are most resistant to corrosion effects in the state of treatment for a solid solution. Tempering in the temperature range of excess phases (carbides, carbonitrides, nitrides) reduces the resistance of steel to intergranular and pitting corrosion. This is due to the emergence around the carbides of zones depleted in chromium, with reduced corrosion resistance. The less (negative) the pitting corrosion potential of an alloy, the greater its tendency to pitting. The value of the pitting potential is a measure of the tendency of metals to pitting.
The works [7, 9, 16, 27 – 31] are dedicated to the studies of the effect of heat treatment on corrosion resistance of steels with 13 % Cr.
In [7] the object of the studies was steel with 13.7 % Cr with increased carbon content (0.497 %), high-purity due to vacuum melting. The effect of microstructure changes at different austenitizing (ТА) temperatures on various corrosion mechanisms was studied. Polarization scanning was carried out in the 0.1 M NaCl + 0.1 M phosphate buffer solution (pH = 7.5). It is demonstrated that the resistance against general corrosion increases with increasing ТА up to 1100 °C due to dissolution of carbides and the associated increase in the chromium content of the alloy matrix. This also leads to better passivation and a thicker internal passive layer rich in chromium. A further increase in ТА does not increase the chromium content and resistance to general corrosion, since all carbides are dissolved. On the other hand, with increasing ТА up to 1100 °C, the carbon content increases, which increases the internal lattice stress and leads to a more defective passive layer, causing a decrease in the resistance to pitting. A further increase in ТА , without affecting the carbon content, increases the grain size. The density of lattice defects in the bulk material decreases, reducing the defectiveness of the passive layer and increasing the resistance to pitting. In contrast, the critical potential shows a contradictory course, increasing up to 1100 °C and decreasing at lower temperatures. A higher pitting potential means less susceptibility to pitting, while a higher critical pitting potential means slower pitting, if any. The authors of [7] note that:
– the research can show that there is not one corrosion resistance, but several different corrosion mechanisms, which are influenced by different microstructure properties;
– the amount of carbon is a critical factor for the pitting corrosion potential;
– alloys with a lower carbon content exhibit different pitting behavior and, given this, the seemingly contradictory results simply refer to different phenomena and are not a contradiction.
A similar study to evaluate the effect of austenitizing temperature and cooling rate (water/air) on corrosion resistance was also conducted on high carbon steel with 13.92 % Cr, 0.42 % C (X46Cr13 (1.4034)) under potentiodynamic polarization in 0.1 M H2SO4 [9]. Heating followed by cooling in water was performed at temperatures: 850 °C (72 h), 900 °C (9 h), 950 °C (90 min), 1000 °C (30 min), 1050, 1100, 1150 °C (15 min), and 1200 °C (10 min). Heating followed by air cooling was performed at 1000 °C (30 min), 1050 and 1100 °C (15 min). It was also noted, as in [7], that austenitization at temperatures of 1100 °C and above leads to a complete dissolution of carbides. The optimal distribution of chromium and carbon in the mixed crystal is ensured. The elimination of the blocking effect of carbides and the higher diffusion rate lead to significant grain enlargement. Decreasing the austenitizing temperature below 1100 °С leaves mixed chromium and iron carbides in the structure, which reduce hardness and corrosion resistance. Temperature-dependent diffusion processes occur during slow air cooling. New carbides form during cooling at the grain boundaries or in the grains themselves and locally remove chromium from the matrix. Second, iron is precipitated from the remaining mixed chromium and iron carbides as solubility drops sharply with temperature. Both processes lead to chromium depletion during air cooling, which is localized mainly on carbides at 1100 °C and on carbides and grain boundaries at 1000 and 1050 °C. Depletion of the chromium content locally worsens the stability of the passive layer, and the resistance to pitting decreases significantly.
In works [16, 27 – 29] the effect of tempering modes on electrochemical corrosion in aqueous NaCl solutions of 13Cr steels with different carbon content was studied.
Experiments on the potentiodynamic polarization in the 3.5 % aqueous NaCl solution of low carbon steel with 0.03 % C and 12.8 % Cr (AISI 410) were performed after quenching from the temperatures in the range from 950 to 1100 °C and quenching from 1050 °C with tempering at 300 – 700 °C [27]. The corrosion rate of AISI 410 steel decreases as the austenitizing temperature increases. The microstructure after austenitizing and tempering is represented by tempered martensite, residual austenite and carbides. The lowest corrosion current density was obtained after tempering at 300 and 400 °C, and the lowest corrosion rate after austenitizing at 1050 °C, quenching and tempering at 600 °C.
The effect of heat treatment on the corrosion behavior of AISI 420 steel (12.10 % Cr, 0.23 % C) in 0.5 M NaCl with pH = 6.26 and electrical conductivity of 49.9 mS/cm was studied on samples in four structural states [28]. In the initial state (A), a continuously cast calibrated rod was considered. Treatment B was annealing at 770 °C for 20 min and cooling with a furnace. Treatment C was 1000 °C, 30 min, martensite quenching in water. Treatment D was tempering at 700 °C, 60 min, cooling in air. The order of samples by corrosion resistance value from higher to lower was established: B > C > D > A. Sample AISI 420 (B) is the most resistant to corrosion, and sample A is the most susceptible to corrosion. Sample C also showed high polarization resistance.
The results of studies [16, 29] of steels close in the chemical composition of the studied steels, heat treatment modes and conclusions made are summarized in Table 7.
The peculiarity of research [30] is that the evolution of microstructure and corrosion behavior of martensitic stainless steel of type 420 with increased carbon content (13.7 % Cr, 0.46 % C, 0.47 % Si, 0.39 % Mn) was studied, tempering of which after austenitizing (950 °С, 1 h, water) was carried out not only at 550 and 700 °С, but also at lower temperatures of 250 and 400 °С (1 h, air), and the potentiodynamic polarization test was conducted not in salt solution, but in the 0.1 M HCl solution at 20 °С. After austenitization and quenching, the metal had a martensitic structure and most of the Cr23C6 carbides dissolved. After tempering at 250 °C some amount of Cr23C6 carbides was found on the grain boundaries. After tempering at 400 °C they were larger and more abundant, and after tempering at 550 °C precipitation of CrC, Cr7C3 and an even greater number of Cr23C6 particles, also at the grain boundaries, were found. After tempering at 700 °С only Cr23C6 carbides were observed, with local corrosion and nucleation of pits near carbides. After all tempering temperatures, pitting corrosion was observed, with the specimen tempered at 250 °C having the highest corrosion resistance and a hardness value of well above 500 HV, and after treatment at 550 °C, general and intergranular corrosion was also observed. The concentration of chromium in the solid solution after different treatments was: 200, 400 °C – >12 %, 550 °C – 10.5 %, 700 °C – ≈11.5 %, i.e. after the last two treatments it was below the critical level. Thus, in contrast to works [16, 29] (see Table 7), a different order of carbide occurrence during tempering can be noted for the studied steel. The temperature of 250 °C is specified as the best choice of tempering temperature, which provides the highest corrosion resistance (high kinetics Epit and low pit growth kinetics). Tempering modes at 550 and 700 °C should be avoided because corrosion resistance reduced due to a large amount of large-size chromium carbides formed at these tempering temperatures.
Table 7. Effect of tempering at 300, 500-550 and 650 – 700 °C on corrosion resistance of steels
with 13 % Cr and 0.31 – 0.38 % C
Main provisions of the paper | Source [29] | Source [16] |
Steel | 13.3 % Cr, 0.31 % C, 0.04 % V, 0.48 % Cu | 13 % Cr, 0.38 % С, 0.3 % V |
Quenching mode | 1020 °C (30 min, quenching in oil) | 1030 °C (45 min, quenching in oil) |
Tempering mode | 300, 550 and 700 °C (2.5 h, cooling in air) | 300, 500 °C and 650 °C (2 h, cooling in air) |
Type of tests | Potentiostatic polarization tests | |
Test medium | 0.1 M NaCl solution | 3.5 % NaCl aqueous solution |
Precipitation in steels during tempering | 300 °C – nanosized ε-Me 3C carbides; 500–550°C – nanosized Me23C6 carbides 650–700 °C – micron or submicron Me23C6 carbides | |
Structure after austenitizing | Austenitizing at 1020–1030 °C did not lead to the complete dissolution of carbides | |
Fine-lath martensite with residual austenite interlayers at the lath boundaries, Cr23C6 carbides | Martensite and Cr23C6 carbides | |
The share of residual austenite decreases with tempering temperature, and after tempering at 550 and 700 °C residual austenite is not observed | Residual austenite is observed only after tempering at 300 °C, and there is no residual austenite after tempering at 500 and 650 °C | |
Effect of austenitizing and tempering at 300 °C on corrosion resistance | In the austenitized state a passive film enriched with chromium is formed. The sample austenitized and tempered at 300 °C shows less current transients, and no sustained pitting corrosion is observed in the 3 h test | Pitting corrosion potential Epit of hardened steel is higher than that of tempered steel and decreases with increasing tempering temperature. Relatively low-temperature tempering (300 °C) slightly reduced corrosion resistance compared to steel after quenching |
Corrosion after tempering at 500–550 °C | Tempering reduced the pitting potential and increased the metastable pitting. Tempering at 550 °C made the steel highly prone to pitting. Pitting occurred at the carbide-matrix interface due to the presence of chromium depletion areas associated with the massive precipitation of chromium-rich carbide. The passive film formed at corrosion potential was enriched with iron particles. It was less protective than the film after austenitization and increased the corrosion current density at corrosion potential and showed no passivity in the 0.1 M NaCl solution above the corrosion potential | The sample after tempering at 500 °C exhibits active corrosion behavior without passivation. This is explained by the precipitation of a large number of chromium-rich nanosized Me23C6 carbides. The large carbide/matrix interface, as pitting occurred, prevented the formation of a protective passive film on the steel surface due to the small distance between the carbides |
Comparison of the corrosion behavior after tempering at 500–550 and 660–700 °C and final conclusion | The Epit value is higher for the sample tempered at 700 °C compared to the sample tempered at 550 °C. A possible reason is the repeated diffusion of Cr from the matrix into the depleted regions, which minimizes the discontinuity of the interfacial regions. The above results confirm the assumption that tempering of steel with 13 % Cr should be carried out at 700 °C because it also provides resistance to pitting. | The sample 1030-650 showed better corrosion resistance than sample 1030-500, even though the Cr content of the matrix was slightly lower than that of sample 1030-500. The tempering temperature for the 13 % Cr steels should be much lower or higher than 500 °C to avoid the massive precipitation of nanosized Me23C6 carbides. Steels with 13 % Cr, tempered at 300 °C, show a combination of high relative hardness and high corrosion resistance. |
Since the AISI 420 martensitic stainless steel is quenched and tempered or double tempered at temperatures up to 250 °C for tableware applications, corrosion resistance was also compared for steel with 12.1 % Cr and 0.19 % C after single and double tempering at 180 °C (2 h, air) after austenitizing at 1050 °C (5 min, air) [31]. The potentiodynamic polarization test was performed in aerated 3.5 % NaCl (рH = 6.0). Single tempering showed a hardness close to air quenching and did not degrade the pitting corrosion resistance. Double tempering did not improve the resistance to pitting corrosion, and hardness decreased afterwards. Only single tempering is recommended.
Conclusions
The properties of steels with 12 – 14 % Cr and 0.2 ≤ % С ≥ 0.4: industrial steels produced with heat treatment according to the standards and known from reference literature, as well as metal properties of laboratory melts treated by various modes of austenitizing and tempering are considered.
In these steels initially annealed at ~800 °C with the formation of the ferrite-carbide structure, with their heating from 800 to 1240 °C, the dissolution of carbides of Me23С6 type occurs, which causes the formation of austenite at 810 – 820 °C with the fixation during quenching of the martensite-carbide structure. Depending on the concentration of carbon in these steels, carbide dissolution in them ends at 950 – 1050 °C. Dissolution of carbides is accompanied by the growth of the austenite grain and preservation of residual austenite after quenching in the structure. Therefore, as the austenitization temperature increases, the quenched steels first show a linear increase in martensite hardness due to carbide hardening (c/a = 0.45[C] + 1.00). And then, when the maximum degree of carbide dissolution is achieved, the steels hardness decreases with further heating, which is associated with the formation of residual austenite and growth of the austenite grain. The maximum effective austenitizing temperature before quenching, which provides high hardness, is 1000 – 1020 °С (HV ~550) for 20Cr13 steels and 1100 – 1120 °С (HV 700 – 750) for 45Cr13 steels.
The grain size during austenitization is the coarser the longer the holding time at a given temperature, and this effect is the more significant the higher the heating temperature. The longer the holding time in the temperature range above the maximum effective austenitizing temperature, the greater the residual austenite in the steel and the lower the hardness after hardening.
The temperature required to achieve complete dissolution of Me23С6 carbides in the austenitic phase increases with the increasing heating rate. High-rate heating leads to a shift of hardening curves after quenching by 40 – 60 °С up the temperature scale compared to the curves obtained during furnace heating.
Quenching not in water at slower cooling rates than 20 K/s (including air) causes precipitation of some carbides.
Hardened steels 20Cr13 – 40Cr13 are characterized by high strength, hardness, and low ductility, especially high-carbon steels. Tempering of hardened laboratory steels in the range up to 400 °C causes a slight decrease in martensite hardness and strength (a small amount of carbides precipitates in martensite, and it becomes unstable). In the interval of 400 – 500 °C a slight increase in hardness and strength due to the effect of dispersion hardening is possible. Then, in the range of 500 – 780 °С, there is a significant decline in these characteristics (intensive precipitation of carbides → decomposition of martensite into ferrite and carbides → coagulation of carbides and their partial dissolution, recrystallization). The plasticity and impact strength increase symmetrically.
The heat treatment with double quenching at 1040 °C + 980 °C provided a finer grain size along with a higher degree of carbon dissolution in the austenitic matrix. During tempering (double at 710 °C + 680 °C), very fine carbides (having a much smaller size compared to the single heat treatment process) formed in small numbers at low-angle and high-angle boundaries. This resulted in increased strength and impact strength after tempering, compared to single quenching from 980 °C.
Corrosion resistance increases with increasing austenitizing heating temperature and decreases with increasing tempering temperature, at which pitting and intergranular corrosion are added to general corrosion, which is associated with the precipitation of Cr23C6 carbides and depletion of the matrix in chromium to the concentrations below 12 %. The recommended heat treatments for 20Cr13 steels are quenching with low tempering at 200 – 300 °C (combination of high strength, good corrosion resistance and satisfactory plasticity), or quenching with high tempering at ~700 °C (good plasticity, satisfactory corrosion resistance). For steels of 40Cr13 type the temperature of ~700 °С is not recommended. The worst tempering temperature is 500 – 550 °С because of the maximum precipitation of ultradispersed carbides.
The possibility of ensuring increased wear resistance of 40Cr13 steels by saturating the surface layer with nitrogen (nitrocementation, ion-plasma nitriding, nitriding and heat treatment), surface laser and plasma quenching, a combination of heat and mechanical treatments, high-vacuum annealing and diffusion siliconizing is demonstrated.
References
1. Metals Handbook. Vol. 8: Metallography, Structures and Phase Diagrams. 8th ed. American Society for Metals; 1973: 465.
2. Ivashko V.V. Influence of heating modes on the structure and properties of stainless steel 20Kh13. Vestnik BarGU. Seriya: Tekhnicheskie nauki. 2015; (3): 45–48. (In Russ.).
3. Scheuer C.J., Fraga R.A., Cardoso R.P., Brunatto S.F. Effects of heat treatment conditions on microstructure and mechanical properties of AISI 420 steel. In: 21 CBECIMAT – Congresso Brasileiro de Engenharia e Ciência dos Materiais 9 a 13 de Novembro de 2014, Cuiabá, MT, Brasil. 2014: 5857–5867.
4. Xiao Li, Yinghui Wei. Effect of austenitising heat treatment on microstructure and properties of a nitrogen bearing martensitic stainless steel. Open Physics. 2019; 17(1): 601–606. https://doi.org/10.1515/phys-2019-0061
5. Garcia de Andrés C., Álvarez L.F., López V. Effects of carbide-forming elements on the response to thermal treatment of the X45Cr13 martensitic stainless steel. Journal of Materials Science. 1998; 33: 4095–4100. https://doi.org/10.1023/A:1004424329556
6. Grinberg E.M., Goncharov S.S., Mova D.A., Kondaurova E.Yu., Surovtseva E.A. Effect of cooling rate during quenching on the structure and hardness of Kh13 steels with different carbon content. Izvestiya TulGU. Tekhnicheskie nauki 2009; (3): 1–11. (In Russ.).
7. Bösing I., Cramer L., Steinbacher M., Werner Zoch H., Thöming J., Baune M. Influence of heat treatment on the microstructure and corrosion resistance of martensitic stainless steel. AIP Advances. 2019; 9(6): 065317. https://doi.org/10.1063/1.5094615
8. Zubchenko A.S., Koloskov M.M., Kashirskii Yu.V., etc. Grader of Steels and Alloys. Moscow: Mashinostroenie; 2003: 784. (In Russ.).
9. Rosemann P., Müller C., Kauss N., Halle T. Einfluss der Wärmebehandlung auf Mikrostruktur und Korrosions- verhalten kohlenstoffhaltiger nichtrostender Stähle. Werkstofftechnischen Kolloquium (Chemnitz), September, 2014: 10.
10. Garcia de Andrés C., Caruana G., Alvarez L.F. Control of M23C6 carbides in 0.45C–13Cr martensitic stainless steel by means of three representative heat treatment parameters. Materials Science and Engineering: A. 1998; 241(1–2):
11. –215. https://doi.org/10.1016/S0921-5093(97)00491-7
12. Behrens B.-A., Hübner S., Sunderkötter C., Gebel L., Gnaß S., Berndt G., Trimborn C., Pfeffer C. Influence of process parameters on the hot stamping of carbon-martensitic chromium steel sheets. IOP Conference Series: Materials Science and Engineering. 2018; 418: 012007. https://doi.org/10.1088/1757-899X/418/1/012007
13. Ma Hou-Yu, He Yin-Sheng, Lee Kwon-Yeong, Shin Keesam. Effect of heat treatment on microstructural evolution of 13Cr martensitic stainless steel. Key Engineering Materials. 2016; 727: 29–35. https://doi.org/10.4028/www.scientific.net/KEM.727.29
14. Abdul Kareem F. Hassan, Qahtan Adnan Jawad. Investigation of the effect of austenitizing temperature and multiple tempering on the mechanical properties of AISI 410 martensitic stainless steel. The Iraqi Journal for Mechanical and Material Engineering. Special Vol. Babylon First Int. Engineering Conf. 2016; C: 411– 435.
15. Abdul Kareem F. Hassan, Qahtan Adnan Jawad. Estimation of austenitizing and multiple tempering temperatures from the mechanical properties of AISI 410 using artificial neural network. International Journal of Engineering & Technology. 2018; 7(4.19): 778–787. https://doi.org/10.14419/ijet.v7i4.19.27997
16. Balan K.P., Venugopal Reddy A., Sarma D.S. Effect of single and double austenitization treatments on the microstructure and mechanical properties of 16Cr-2Ni steel. Journal of Materials Engineering and Performance. 1999; 8(3): 385–393. https://doi.org/10.1361/105994999770346963
17. Lu S.-Y., Yao K.-F., Chen Y.-B., Wang M.-H., Liu X., Ge X. The effect of tempering temperature on the microstructure and electrochemical properties of a 13 wt.% Cr-type martensitic stainless steel. Electrochimica Acta. 2015; 165: 45–55. https://doi.org/10.1016/j.electacta.2015.02.038
18. Kulkarni S., Srinivas P., Biswal P.K., Balachandran G., Balasubramanian V. Improvement in mechanical properties of 13Cr martensitic stainless steels using modified heat treatments. In: Proceedings of the 28th ASM Heat Treating Society Conference. Detroit, 2015: 335–341.
19. Mohameda Hareer S., Ataiwib Ali H., Dawood Jamal J. Mechanical properties of martensitic stainless steel (AISI420) subjected to conventional and cryogenic treatments. Engineering and Technology Journal. 2020; 38 A(8): 1096–1105. https://doi.org/10.30684/etj.v38i8A.517
20. Romanenko D.N., Nikulin A.A., Gadalov V.N., etc. Prospects for the use of stainless steel 40Kh13 for knives of meat-grinding equipment. Konstruktsii iz kompozitsionnykh materialov. 2011; (4): 59–63. (In Russ.).
21. Rutkovskii A.V. Kumurzhi A.Yu. Wear resistance of 40Kh13 steel after hardening by thermal cyclic ion-plasma nitriding under abrasive wear. Problems of Friction and Wear. 2012; 57: 240–250. (In Russ.). https://doi.org/10.18372/0370-2197.57.3607
22. Sidelev D.V., Voronina E.D., Kozhina O.I., Grudinin V.A., Stolbovskaya G.N. Nitriding of 40H13 steel in inductively coupled plasma: Role of a bias potential. Prikladnaya fizika. 2022; (2): 16–23. (In Russ.).
23. Kukareko V.A., Kushnerov A.V. Influence of pre-heat treatment on wear resistance of 40Kh13 steel modified with nitrogen ions. Uprochnyayushchie tekhnologii i pokrytiya. 2022; 18(2(206)): 61–65. (In Russ.). https://doi.org/10.36652/1813-1336-2022-18-2-61-65
24. Korostelev V.F., Kirilina A.N. Analysis of hardening of blade tools from steel 40Kh13 due to laser heating. Metallovedenie i termicheskaya obrabotka metallov. 2011; (3(669)): 38–41. (In Russ.).
25. Belinin D.S., Shchitsyn Yu.D. Features of structure formation during plasma surface hardening to a great depth of products from steel 40Kh13. Izvestiya Samarskogo nauchnogo tsentra Rossiiskoi akademii nauk. 2012; 14(4-5): 1202–1205. (In Russ.).
26. Suslov A.G., Shalygin M.G. Comprehensive technological increase in wear resistance and static strength of parts made of steel 40Kh13. Naukoemkie tekhnologii v mashinostroenii. 2018; (1(79)): 19–21. (In Russ.).
27. Grigorchik A.N., Belotserkovskii M.A., Kukareko V.A. Wear resistance of an ion-nitrided gas-thermal coating of martensitic steel 40Kh13. In: Abstracts of the Int. Sci. and Tech.Conf. “POLIKOMTRIB-2017”, Gomel’, 27–30.06.2017: 130. (In Russ.).
28. Rizky D., Syaiful A.M., Rusnaldy, Mabruri E. Effect of heat treatment of AISI 410 martensitic stainless steel on microstructure and corrosion resistance. Metalurgi (Indonesia). 2018; 33(1): 18–24.
29. Minciuna M.G., Achitei D.C., Vizureanu P., Benchea M., Sandu A.V. The effect of heat treatment and corrosion behavior of AISI 420. IOP Conference Series: Materials Science and Engineering. 2018; 374: 012039. https://doi.org/10.1088/1757-899X/374/1/012039
30. Bonagani S.K., Bathula V., Kain V. Influence of tempering treatment on microstructure and pitting corrosion of 13 wt.% Cr martensitic stainless steel. Corrosion Science. 2018; 131: 340–354. https://doi.org/10.1016/j.corsci.2017.12.012
31. Zhou Y., Engelberg D.L. Accessing the full spectrum of corrosion behaviour of tempered type 420 stainless steel. Materials and Corrosion. 2021; 72(11): 1718–1729. https://doi.org/10.1002/maco.202112442
32. De Alcântara C.M., de Moura A.N., D’Azeredo Orlando M.T., da Silva Labiapari W., da Cunha M.A., de Oliveira T.R., Lopes Buono V.T. Microstructure and pitting corrosion resistance of quenched, single tempered and double tempered AISI 420 martensitic stainless steel. Materials Research. 2021; 24(6): 20210093. https://doi.org/10.1590/1980-5373-MR-2021-0093
About the Authors
M. V. KostinaRussian Federation
Mariya V. Kostina, Dr. Sci. (Eng.), Assist. Prof., Senior Researcher, Head of the Laboratory “Physicochemistry and Mechanics of Metallic Materials”
49 Leninskii Ave., Moscow 119991, Russian Federation
L. G. Rigina
Russian Federation
Lyudmila G. Rigina, Cand. Sci. (Eng.), Leading Researcher, Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences; JSC Russian State Research Center “CNIITMASH”
49 Leninskii Ave., Moscow 119991, Russian Federation
4 Sharikopodshipnikovskaya Str., Moscow 115088, Russian Federation
V. S. Kostina
Russian Federation
Valentina S. Kostina, Cand. Sci. (Eng.), Junior Researcher of the Laboratory “Physicochemistry and Mechanics of Metallic Materials”
49 Leninskii Ave., Moscow 119991, Russian Federation
A. E. Kudryashov
Russian Federation
Aleksandr E. Kudryashov, Research Engineer
49 Leninskii Ave., Moscow 119991, Russian Federation
R. S. Fedortsov
Russian Federation
Ruslan S. Fedortsov, Research Engineer
49 Leninskii Ave., Moscow 119991, Russian Federation
Review
For citations:
Kostina M.V., Rigina L.G., Kostina V.S., Kudryashov A.E., Fedortsov R.S. Corrosion-resistant steels based on Fe – ~13 % Cr: Heat treatment, corrosion- and wear resistance. Review. Izvestiya. Ferrous Metallurgy. 2023;66(1):8-26. https://doi.org/10.17073/0368-0797-2023-1-8-26